Supplementary Information (docx 3748K)
advertisement

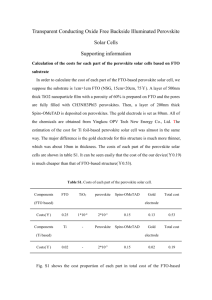
Supporting Information Highly stable and efficient solid state solar cells based on methylammonium lead bromide (CH3NH3PbBr3) perovskite quantum dots Sawanta S. Mali Chang Su Shim, Chang Kook Hong* *Polymer Energy Materials Laboratory, School of Applied Chemical Engineering, Chonnam National University, Gwangju, 500-757 (South Korea), S1 Characterizations The surface morphology of the prepared samples was recorded by a field emission scanning electron microscope (FESEM; S-4700, Hitachi). Transmission electron microscopy (TEM) micrographs, selected area electron diffraction (SAED) pattern and high-resolution transmission electron microscopy (HRTEM) images were obtained by TECNAI F20 Philips operated at 200 KV. The TEM sample was prepared by drop casting of ethanolic dispersion of the sample onto a carbon coated Cu grid. X-ray diffraction (XRD) measurements were carried out using a D/MAX Uitima IIIXRD spectrometer (Rigaku, Japan) with CuK line of 1.5410 Å. The elemental information regarding the deposited samples was analyzed using an STEM and EDS analysis which is connected with TEM. The cells were illuminated using a solar simulator at AM 1.5 G for 10 s, where the light intensity was adjusted with an NREL-calibrated Si solar cell with a KG-5 filter to 1 sun intensity (100 mW cm-2). The IPCE spectra were measured as a function of wavelength from 300 to 1100 nm on the basis of a Spectral Products DK240 monochromator. Photoluminescence measurements were carried out on a PL mapper (Accent Opt. Tech. UK, Model:RPM 2000, 532nm ND-YAG laser excitation). Figure S1 Plane view of STEM micrographs of CH3NH3PbBr3 decorated mp-TiO2 and EDS mapping of each elements. The elements are mentioned as per respective colors. Carbongreen, titanium-red, oxygen-cyan, lead-yellow, bromine-cyan. Figure S2 TEM analysis of CH3NH3PbBr3+mp-TiO2 composite having ~10nm CH3NH3PbBr3 particle size (a) TEM micrograph of the perovskite CH3NH3PbBr3 deposited on mp-TiO2 nanoparticles (b-c) Highly magnified TEM images of CH3NH3PbBr3 coated TiO2 nanoparticles at different magnification. (d) HRTEM image of CH3NH3PbBr3 +mpTiO2. Figure S3 TEM analysis of CH3NH3PbBr3+mp-TiO2 composite having ~7-6nm particle size (a) TEM micrograph of the perovskite CH3NH3PbBr3 deposited on mp-TiO2 nanoparticles (b-c) Highly magnified TEM images of CH3NH3PbBr3 coated TiO2 nanoparticles at different magnification. Inset shows FFT analysis of single CH 3NH3PbBr3 nanoparticle (d) HRTEM image of CH3NH3PbBr3 +mpTiO2. Figure S4 TEM analysis of CH3NH3PbBr3+mp-TiO2 composite having ~5-4nm CH3NH3PbBr3 particle size (a) TEM micrograph of the perovskite CH 3NH3PbBr3 deposited on mp-TiO2 nanoparticles (b-c) Highly magnified TEM images of CH3NH3PbBr3 coated TiO2 nanoparticles at different magnification. (d) HRTEM image of CH3NH3PbBr3 +mpTiO2. Figure S5 Normalized photoluminescence spectra of MAPbBr3 nanoparticles/quantum dots with different size. MAPbBr3 (~10nm) MAPbBr3 (~7nm) MAPbBr3 (~5nm) Normalized PL MAPbBr3 (~3nm) 550 600 Wavelength (nm) 650 700 Figure S6 J-V curves of forward and reverse bias sweep and respective JV curves for spiroMeOTAD using CH3NH3PbBr3 perovskite absorber layer with different size. J-V curves measured by forward and reverse scans with 10mV voltage steps and 50ms delay times under AM 1.5 G illumination. 12 10nm Forwad 10nm Reversed 7nm Forward 7nm Reversed 5nm Forward 5nm Reversed -2 Current density (mA.cm ) 10 8 6 4 2 FTO/Bl-TiO2/mp-TiO2+MAPbBr3/spiro-MeOTAD/Au 0 0.0 0.2 0.4 0.6 Voltage (V) 0.8 1.0 Figure S7 J-V curves of forward and reverse bias sweep and respective JV curves for PTAA using CH3NH3PbBr3 perovskite absorber layer with different size. J-V curves measured by forward and reverse scans with 10mV voltage steps and 50ms delay times under AM 1.5 G illumination. Current density (mAcm-2) 12 10nm Forward 10 reversed 7nm Forward 7nm Reversed 5 nm Forward 5nm Revesred 10 8 6 4 2 FTO/Bl-TiO2/mp-TiO2+MAPbBr3/PTAA/Au 0 0.0 0.2 0.4 0.6 0.8 Voltage (V) 1.0 1.2 Figure S8 Cross-sectional field emission scanning electron microscopic images of FTO/BlTiO2/mp-TiO2+MAPbBr3/HTM/Au. (a-d) cross sectional images of PTAA based devices (e-f) spiro-MeOTAD based perovskite devices. The mp-TiO2 layer has been deposited at different spin coating speed. (a) 2500, (b) 3000, (c) 4000, (d) 5000, (e) 3000 (f) 4000rpm. Figure (e) (e) and (f) show spiro-MeOTAD HTM based devices. Figure (e) shows ~80nm Au contact. Figure S9 Average solar cell efficiencies were obtained from different MAPbBr3 nanoparticles/quantum dots with different HTM materials. 8 10 spiro-MeOTAD PTAA 9 8 6 7 5 6 5 4 4 3 2 4 6 MAPbBr3 size (nm) 8 10 Efficiency for PTAA Efficiency for spiro-MeOTAD 7 Table S1: Solar cell properties of MAPbBr3 based perovskite solar cells having different size. Device configuration FTO/Bl-TiO2/mp-TiO2/CH3NH3PbBr3/spiro-MeOTAD/Au HTM SpiroMeOTAD SpiroMeOTAD SpiroMeOTAD Particle Scan size direction ~10nm ~7-8nm ~5-7nm VOC (V) JSC (mAcm-2) FF () Average Forward 0.839 8.37 0.43 3.02 3.47 Reverse 0.888 8.15 0.54 3.91 Forward 0.904 9.11 0.49 4.04 Reverse 0.873 8.97 0.54 4.22 Forward 0.862 10.06 0.46 4.45 Reverse 0.894 9.79 0.52 4.55 4.13 4.5 Table S2: Solar cell properties of MAPbBr3 based perovskite solar cells having different size. Device configuration FTO/Bl-TiO2/mp-TiO2/CH3NH3PbBr3/PTAA/Au HTM PTAA PTAA PTAA Particle Scan size direction ~10nm ~7-8nm ~5-7nm VOC (V) JSC (mAcm-2) FF () Average Forward 1.071 8.10 0.42 3.64 4.02 Reverse 1.069 8.41 0.49 4.40 Forward 1.043 09.21 0.52 4.99 Reverse 1.047 09.44 0.58 5.73 Forward 1.032 10.62 0.53 5.81 Reverse 1.082 10.85 0.59 6.93 5.36 6.73