jssc4434-sup-0001
advertisement

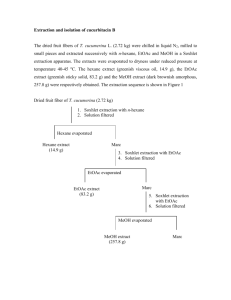
Fundamental studies on the feasibility of deep eutectic solvents for the selective partition of glaucarubinone present in the roots of Simarouba glauca Faisal Kholiya, Nidhi Bhatt, Meena R Rathod, Ramavatar Meena and Kamalesh Prasad * CSIR-Central Salt and Marine Chemicals Research Institute, G. B. Marg, Bhavnagar-364002 (Gujarat), India. Email: kamlesh@csmcri.org; drkamaleshp@gmail.com; Phone No.: +91278-2567760. Fax: +91-278-2567562. Details of extraction of glaucarubinone using conventional solvent extraction method The active extract i.e., the alcohol: water 95:05 extract of root, which yielded 08-11% on basis of dry material, was fractionated on Automated Combi Flash Separation System into 6 fractions. The first fraction (F1) was obtained through extraction with neat hexane, the residue was subjected to extract with neat chloroform to yield the second fraction (F2), residue was then subjected to extract with 90:10 chloroform: methanol to yield a third fraction (F3), residue was then subjected to extract with 1:1 chloroform: methanol to yield a fourth fraction (F4), the residue was then extracted with neat methanol to yield a fifth fraction (F5) and the residue was then treated with pure water to obtain a sixth fraction (F6) after filtration. Yields of F1, F2, F3, F4, F5 and F6 were 6%, 15%, 3%, 56%, 16% and 1% respectively with 3.0% process loss. The summary of the extraction is depicted below 1 Experimental section : Conventional solvent extraction method employed to extract glaucarubinone was described in the experimrntal section of the main text (Scheme 1). For fractionation of crtude Automated Combi Flash Separation System (Isco, UA-6 ). Semi preparative HPLC system (Shimadzu , LC 8A) was used to purify the individual components of the fractions. For the purpose RP-18 column (250 x 21.20 mm, 10µ micro, Desc.: Luna 10µ C18(2)) was used as stationary phase with 59:41 methanol: water as mobile phase under isocratic conditions. The detection wavelength was 220 nm with flow rate 9.00 ml/min and run period of 30 min was maintained throughout the experiments. Each injection contained about 13-15 mg of the sub-fractions. The flow chart of extraction is depicted below. Figure S1 : 1H NMR spectra for glaucarubinone (in CDCl3) extracted using conventional solvent extraction method. 2 Figure S2 : HPLC profile for pure glaucarubinone dissolved in water. Figure S3 : HPLC profile for pure glaucarubinone dissolved in Chol.Cl-Urea 1:2. 3 Figure S4 : HPLC profile of only Chol.Cl-Urea 1:2 diluted with water (1: 10). Figure S5 : HPLC profile for glaucarubinone rich extract in Chol.Cl-Urea 1:2. 4 Figure S6 : ESI-MS spectra of pure glaucarubinone extracted from Simaruba glauca employing conventional solvent extraction method. Figure S7: ESI-MS/MS spectra of pure glaucarubinone extracted from Simaruba glauca employing conventional solvent extraction method at m/z of 495.32. 5 Figure S8 : ESI-MS spectra for Chol.Cl-Urea 1:2 extract of glaucarubinone rich extract of Simarouba glauca. Figure S9 : ESI-MS/MS spectra for Chol.Cl-Urea 1:2 extract of glaucarubinone rich extract of Simarouba glauca fragmented at m/z of 495.46. 6