Branchial anomalies: A Retrospective analysis in a tertiary care
advertisement

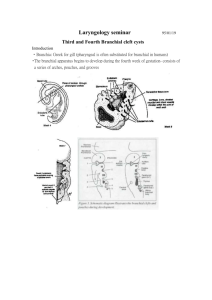
Branchial anomalies: A Retrospective analysis in a tertiary care hospital. Abstract Branchial anomalies are the second most common congenital head and neck masses. Although congenital, all age groups are known to be affected. Branchial anomalies are common congenital pediatric head and neck lesions but are comprised by several diverse anomalies. Treatment must be tailored depending on which branchial arch is involved and whether a cyst mass or sinus/fistula tract is present. Keywords Branchial anomaly, branchial cleft cyst, branchial fistula, branchial sinus, congenital neck mass, pediatric neck mass INTRODUCTION Branchial anomalies comprise approximately 20% of pediatric congenital head and neck masses, making them, after thyroglossal duct cysts, the second most common1. Although the term branchial anomaly encompasses first, second, third and fourth branchial cleft cysts, sinuses and fistulae, all of which are distinct clinical entities and require separate management, all are thought to result from a similar embryologic error: incomplete obliteration of the branchial apparatus during embryogenesis2. The branchial apparatus consists of six grooves (ectoderm), arches (mesoderm) and pouches (endoderm). When a pouch or groove fails to obliterate, it may communicate with either skin or mucosa of the upper airway, forming a sinus. When both a pouch and a groove fail to obliterate, it may form a communication between the skin and mucosa, which is termed a fistula. When a branchial groove remnant forms an epithelial-lined space without communication to the skin or mucosa, a cyst is formed1. Each of the six branchial arches (with the exception of the fifth) develops into specific structures in the head and neck. A branchial anomaly and its associated tract typically lies inferior to all the derivatives of its associated arch and superior to all derivatives of the next arch2. The malformations can, therefore, be divided into first through fourth branchial anomalies. PATIENTS AND METHODS: A retrospective analysis was conducted between Jan 2008 to June 2012, over a period of 54 months in KIMS Bangalore. Total number of 31 cases were analysed. RESULTS First and second decade was the most common age group affected. (Chart 1). Eighty nine percent of patients were males. Right sided lesions were seen in 68% of patients, left in 22% and bilaterally in 10% of patients. Discharge from the external opening and swelling were the most common symptoms. (Figures 1, 2, and 3). Surgery was performed in all the patients. Elliptical incision was used in the majority of cases; step ladder incision was the next most common incision used. (Figure 4). DISCUSSION FIRST BRANCHIAL ANOMALY First branchial clefts comprise 5–25% of all branchial anomalies3,4. Their location is variable, extending from the retroauricular and parotid region, cervically from the area below the mandible and above the hyoid5. Symptoms at presentation typically vary with location. Parotid and retroauricular lesions classically present as an enlarging mass often after infection with associated erythema and pain. Occasionally, otorrhea may be seen from the external auditory canal6,7. When within the parotid, the course of the tract in relationship to the facial nerve is variable8. Work9 classified first branchial cleft anomalies on the basis of anatomical and histological features. Type 1 is of ectodermal origin and is considered a duplication of the membranous external auditory canal. Typically, they present in young children as a thin walled, soft mass protruding in the external auditory canal. Type 2 is a duplication of the membranous external auditory canal and pinna, containing skin (ectoderm) and cartilage (mesoderm). Classically, they present later in childhood as a cyst, sinus or fistula5. SECOND BRANCHIAL ANOMALY Second clefts are the most common, ranging from 40 to 95% of all branchial anomalies1,10,11. Classically, their complete course begins near the anterior border of the sternocleidomastoid muscle (SCM), tracks superior and lateral to the common carotid artery, passing medially between the external and internal carotid arteries, passing lateral and superior to the glossopharyngeal and hypoglossal nerves and penetrating the middle pharyngeal constrictor muscle to open into the tonsillar fossa10, 12. Sinuses are more frequent than cysts, which are more frequent than fistulae13,14. Second branchial cysts classically present as a painless neck mass along the anterior border of the upper third of the SCM, or after acute enlargement with concomitant respiratory infection15. Sinuses typically present inferiorly with a draining opening near the base of the neck where the strap muscles meet the SCM. Unilateral fistulae are found much more commonly on the right side with rates reported as high as 89%, whereas bilateral anomalies may be associated with branchiootorenal syndrome16. THIRD BRANCHIAL ANOMALY Third clefts represent only 2–8% of all branchial malformations4,17. Similar to second anomalies, the tract arises at the skin along the mid to lower third of the anterior border of the SCM, pierces the platysma with variable passage through the thyroid. It then ascends along the carotid sheath, passing over the superior laryngeal nerve, deep to the glossopharyngeal nerve, behind the internal carotid, piercing the thyrohyoid membrane and entering the upper lateral piriform sinus wall18,19. In contrast to second branchial sinuses, third clefts are predominately on the left, with a reported rate of 89%20. The classic presentation is as a recurrent left abscess or acute suppurative thyroiditis during the first decade of life20,21. FOURTH BRANCHIAL ANOMALY The theoretic course of a fourth branchial anomaly is similar to a third except it passes under the superior laryngeal nerve but over the recurrent laryngeal and hypoglossal nerves. It then dips back into the chest to pass around the aortic arch on the left and the subclavian artery on the right. It then ascends to enter the larynx near the cricothyroid joint or through the lower horn of the thyroid cartilage, through the inferior constrictor, and into the apex of the piriform sinus19,22. The key difference between the third and fourth is the relationship to the superior and recurrent laryngeal nerves. The third will pass superficial to both the superior and recurrent where the fourth will pass deep to the superior but superficial to the recurrent. These anomalies, as in the third, are predominately on the left side, with Nicoucar et al23 reporting 93.5% left-sided10. DIAGNOSTIC INVESTIGATIONS CT is the most commonly used radiograph by practitioners1,15,20,23 and used to diagnose cystic lesions. ULTRASOUND Ultrasound is easy, fast and does not require sedation or radiation. Branchial cysts appear as well defined, smoothly outlined and uniformly anechoic lesions with posterior enhancement24. Studies of diagnostic accuracy vary. Mitroi et al13 report 100% accuracy in the diagnosis of 23 branchial cysts and sinuses, thus, obviating the need of CT or MRI. Others report significantly lower accuracy citing operator dependency as a principal cause25,26. Ultrasound is limited in its ability to depict hypopharyngeal lesions when compared with CT or MRI25. Nicoucar et al20 reported a positive predictive value for ultrasound of only 7% for third branchial anomalies. COMPUTED TOMOGRAPHY AND MRI A review of 87 patients who received a preoperative CT for a reported branchial anomaly revealed a diagnostic accuracy of 95% for cysts, 81% for sinuses and 50% for fistulae1. Several radiologic features of contrast-enhanced CT are typical of branchial clefts including fat streaking and hypodensity within the ipsilateral thyroid lobe, and gas bubbles along the tract or within the cyst27. Unfortunately, the ability of CT to chart the entire course of a sinus tract or fistula is variable. In first branchial anomalies, high-resolution CT effectively shows the relationship with the external auditory canal and the middle ear6. MRI is also accurate in detecting branchial cysts but is variable in its effectiveness in delineation of the fistula or sinus tract28,29,30. In regards to first branchial anomalies, MRI allows assessment of the extent of the lesion, especially within parotid tissue6. Chan et al.7 reported that the relationship of the facial nerve and the deep portion of the tract was difficult to establish preoperatively in first branchial anomalies with both MRI and CT. Nicoucar et al.20,23 report a positive predictive value for MRI and CT of 84 and 49% for third branchial anomalies and 63 and 46% for fourth branchial anomalies, respectively. Fistulography, barium swallow, direct laryngoscopy/pharyngoscopy are other investigative modalities. TREATMENT Historically, definitive treatment of branchial anomalies has been complete surgical excision. These lesions do not spontaneously regress and often are subject to recurrent infections. The timing of surgery is controversial and dependent on patient age and infection history. Some1 recommend early surgical excision at 1 year of age, ideally performed before infections occur and distort the surgical planes of dissection. Support to this model is given by the increased rate of recurrence of previously infected anomalies4,37. This is in contrast to others’ recommendation of delaying surgery until age 2– 34,17, when adjacent structures are larger and easier to identify. FIRST BRANCHIAL ANOMALIES The ideal surgical approach is a superficial parotidectomy with facial nerve dissection and complete excision of the lesion1,2, due to the potential intimate and variable course of the tract with the facial nerve. In D’Souza’s et al8 literature review, of 87 cases of excision with facial nerve identification, 21% resulted in temporary nerve paralysis and less than 1% with permanent paralysis. This is compared with 29 and 12%, respectively, in cases in which the facial nerve was not identified. Case reports of less invasive surgical techniques that bypass parotidectomy have been reported with acceptable results5,7,38. SECOND BRANCHIAL ANOMALIES The traditional treatment of second branchial anomalies is surgical excision of the entire lesion. This involves a transverse cervical incision encompassing the external sinus opening when present. During cyst excision, careful exploration for an associated sinus tract must be performed15. During sinus excision, a second ‘step-laddered’ incision may be required for better exposure near the pharynx10,21. Fistula excision may be aided with cannulation of the tract with 2–0 or 3–0 monofilament suture or lacrimal probe15. A sinus tract may be injected externally with methylene blue, however, care must be taken as extravasation into surrounding tissues may make dissection more difficult39. Exploration of the carotid sheath to the lateral pharyngeal wall may be undertaken to fully excise any tract that may not have been identified on preoperative imaging1. Approaches offering less visible scarring have been described. Roh and Yoon11 approach second branchial anomalies via a retroauricular incision, which allows excellent visualization with ‘invisible’ external scarring. Endoscopic excision of second cysts via a retroauricular approach may be employed, eliminating nearly all visible scarring40. THIRD AND FOURTH BRANCHIAL ANOMALIES Treatments for third and fourth branchial anomalies have historically been by complete surgical excision of the entire tract with thyroid lobectomy when involved [10&,19,22,23]. Madana et al.21 reported no recurrences with 1–3-year follow-up after excision of 18 lesions with hemithyroidectomy. Pereira et al.19 describe a combined approach including direct laryngoscopy with catheter insertion into the piriform defect prior to formal external surgical excision with no complications or recurrences. A literature review by Nicoucar et al.20,23 of third and fourth branchial anomalies revealed a recurrence rate of 94 and 89%, respectively, after incision and drainage alone. The reported recurrence rate after primary excision alone was 15% for both third and fourth fistulae. When combined with hemithyroidectomy, recurrence rate remained the same for third fistulae, but dropped to 8% for fourth. The complication rate for both lesions was significantly higher when performed on children under 8 years of age. Complete excision is challenging due to their complex anatomical course10,41. Care must be taken to preserve the recurrent laryngeal nerve (RLN), which is often intimately associated with the tract and easily injured22,27,42. Endoscopic techniques have shown successful outcomes with possible reduction of complications including RLN paralysis. These aim to seal the piriform defect using several modalities including electrocoagulation with diathermy probe43,44, low-power diode laser45, chemical cauterization with trichloroacetic acid46,47 and silver nitrate stick48 and fibrin glue49. Leboulanger et al.50, in their review of 20 patients with fourth branchial anomalies treated with laser endoscopic cauterization, report low failure rates (13%) except in neonates (40%). Nicoucar’s et al. review20,23 reports that the recurrence rate for endoscopic procedures was 18 and 15% for third and fourth branchial anomalies respectively, which is almost identical to open excision. No complications were reported. CONCLUSION Branchial anomalies are common congenital pediatric head and neck lesions but are comprised by several diverse anomalies. Treatment must be tailored depending on which branchial arch was involved and whether a cyst mass or sinus/fistula tract is present. References 1. Schroeder JW Jr, Mohyuddin N, Maddalozzo J. Branchial anomalies in the pediatric population. Otolaryngol Head Neck Surg 2007; 137:289–295. 2. Bajaj Y, Tweedie D, Ifeacho S, et al. Surgical technique for excision of first branchial cleft anomalies: how we do it. Clin Otolaryngol 2011; 36:371–374. 3. Olsen KD, Maragos NE, Weiland LH. First branchial cleft anomalies. Laryngoscope 1980; 90:423–436. 4. Choi SS, Zalzal GH. Branchial anomalies: a review of 52 cases. Laryngoscope 1995; 105 (9 Pt 1):909–913. 5. Chen Z, Wang Z, Dai C. An effective surgical technique for the excision of first branchial cleft fistula: make-inside-exposed method by tract incision. Eur Arch Otorhinolaryngol 2010; 267:267–271. 6. Triglia JM, Nicollas R, Ducroz V, et al. First branchial cleft anomalies: a study of 39 cases and a review of the literature. Arch Otolaryngol Head Neck Surg 1998; 124:291–295. 7. Chan KC, Chao WC, Wu CM. Surgical management of first branchial cleft anomaly presenting as infected retroauricular mass using a microscopic dissection technique. Am J Otolaryngol 2012; 33:20–25. 8. D’Souza AR, Uppal HS, De R, Zeitoun H. Updating concepts of first branchial cleft defects: a literature review. Int J Pediatr Otorhinolaryngol 2002; 62:103–109. 9. Work WP. Newer concepts of first branchial cleft defects. Laryngoscope 1972; 82:1581–1593. 10. Bajaj Y, Ifeacho S, Tweedie D, et al. Branchial anomalies in children. Int J Pediatr Otorhinolaryngol 2011; 75:1020–1023. 11. Roh JL, Yoon YH. Removal of pediatric branchial cleft cyst using a retroauricular hairline incision (RAHI) approach. Int J Pediatr Otorhinolaryngol 2008; 72:1503–1507. 12. Olusesi AD. Combined approach branchial sinusectomy: a new technique for excision of second branchial cleft sinus. J Laryngol Otol 2009; 123:1166–1168. 13. Mitroi M, Dumitrescu D, Simionescu C, et al. Management of second branchial cleft anomalies. Rom J Morphol Embryol 2008; 49:69–74. 14. Karabulut R, Sonmez K, Turkyilmaz Z, et al. Second branchial anomalies in children. ORL J Otorhinolaryngology Relat Spec 2005; 67:160–162. 15. Acierno SP, Waldhausen JH. Congenital cervical cysts, sinuses and fistulae. Otolaryngol Clin North Am 2007; 40:161–176. 16. Maddalozzo J, Rastatter JC, Dreyfuss HF, et al. The second branchial cleft fistula. Int J Pediatr Otorhinolaryngol 2012; 76:1042– 1045. 17. Ford GR, Balakrishnan A, Evans JN, Bailey CM. Branchial cleft and pouch anomalies. J Laryngol Otol 1992; 106:137–143. 18. Nusbaum AO, Som PM, Rothschild MA, Shugar JM. Recurrence of a deep neck infection: a clinical indication of an underlying congenital lesion. Arch Otolaryngol Head Neck Surg 1999; 125:1379–1382. 19. Pereira KD, Losh GG, Oliver D, Poole MD. Management of anomalies of the third and fourth branchial pouches. Int J Pediatr Otorhinolaryngol 2004; 68:43–50. 20. Nicoucar K, Giger R, Jaecklin T, et al. Management of congenital third branchial arch anomalies: a systematic review. Otolaryngol Head Neck Surg 2010; 142:21–28; e2. 21. Madana J, Yolmo D, Kalaiarasi R, et al. Recurrent neck infection with branchial arch fistula in children. Int J Pediatr Otorhinolaryngol 2011; 75:1181–1185. 22. Nicollas R, Ducroz V, Garabedian EN, Triglia JM. Fourth branchial pouch anomalies: a study of six cases and review of the literature. Int J Pediatr Otorhinolaryngol 1998; 44:5–10. 23. Nicoucar K, Giger R, Pope HG Jr, et al. Management of congenital fourth branchial arch anomalies: a review and analysis of published cases. J Pediatr Surg 2009; 44:1432–1439. 24. Baatenburg de Jong RJ, Rongen RJ, Lameris JS, et al. Evaluation of branchiogenic cysts by ultrasound. ORL J Otorhinolaryngology Relat Spec 1993; 55:294–298. 25. Park SW, Han MH, Sung MH, et al. Neck infection associated with pyriform sinus fistula: imaging findings. AJNR Am J Neuroradiol 2000; 21:817– 822. 26. Shrime M, Kacker A, Bent J, Ward RF. Fourth branchial complex anomalies: a case series. Int J Pediatr Otorhinolaryngol 2003; 67:1227–1233. 27. James A, Stewart C, Warrick P, et al. Branchial sinus of the piriform fossa: reappraisal of third and fourth branchial anomalies. Laryngoscope 2007; 117:1920–1924. 28. Black CJ, O’Hara JT, Berry J, Robson AK. Magnetic resonance imaging of branchial cleft abnormalities: illustrated cases and literature review. J Laryngol Otol 2010; 124:213–215. 29. Mukherji SK, Fatterpekar G, Castillo M, et al. Imaging of congenital anomalies of the branchial apparatus. Neuroimaging Clin N Am 2000; 10:75–93. 30. Sun Z, Fu K, Zhang Z, et al. Multidetector computerized tomographic fistulography in the evaluation of congenital branchial cleft fistulae and sinuses. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113:688–694. 31. Guarisco JL, Fatakia A. Intraoperative fistulograms in the management of branchial apparatus abnormalities in children. Int J Pediatr Otorhinolaryngol 2008; 72:1777–1782. 32. Seki N, Himi T. Retrospective review of 13 cases of pyriform sinus fistula. Am J Otolaryngol 2007; 28:55–58. 33. Whetstone J, Branstetter BF 4th, Hirsch BE. Fluoroscopic and CT fistulography of the first branchial cleft. AJNR Am J Neuroradiol 2006; 27:1817–1819. 34. Ryu CW, Lee JH, Lee HK, et al. Clinical usefulness of multidetector CT fistulography of branchial cleft fistula. Clin Imaging 2006; 30:339–342. 35. Thomas B, Shroff M, Forte V, et al. Revisiting imaging features and the embryologic basis of third and fourth branchial anomalies. AJNR Am J Neuroradiol 2010; 31:755–760. Pereira KD, Davies JN. Piriform sinus tracts in children. Arch Otolaryngol Head Neck Surg 2006; 132:1119–1121. 37. Reiter D. Third branchial cleft sinus: an unusual cause of neck abscess. Int J Pediatr Otorhinolaryngol 1982; 4:181–186. 38. Baatenburg de Jong RJ. A new surgical technique for treatment of preauricular sinus. Surgery 2005; 137:567–570. 39. Waldhausen JH. Branchial cleft and arch anomalies in children. Semin Pediatr Surg 2006; 15:64–69. 40. Chen LS, Sun W, Wu PN, et al. Endoscope-assisted versus conventional second branchial cleft cyst resection. Surg Endosc 2012; 26:1397–1402. 41. Yang C, Cohen J, Everts E, et al. Fourth branchial arch sinus: clinical presentation, diagnostic workup, and surgical treatment. Laryngoscope 1999; 109:442–446. 42. Narcy P, Aumont-Grosskopf C, Bobin S, Manac’h Y. Fistulae of the fourth endobranchial pouch. Int J Pediatr Otorhinolaryngol 1988; 16:157–165. 43. Jordan JA, Graves JE, Manning SC, et al. Endoscopic cauterization for treatment of fourth branchial cleft sinuses. Arch Otolaryngol Head Neck Surg 1998; 124:1021–1024. 44. Verret DJ, McClay J, Murray A, et al. Endoscopic cauterization of fourth branchial cleft sinus tracts. Arch Otolaryngol Head Neck Surg 2004; 130:465–468. 45. Sayadi SJ, Gassab I, Dellai M, et al. [Laser coagulation in the endoscopic management of fourth branchial pouch sinus]. Ann Otolaryngol Chir Cervicofac 2006; 123:138–142. 46. Stenquist M, Juhlin C, Astrom G, Friberg U. Fourth branchial pouch sinus with recurrent deep cervical abscesses successfully treated with trichloroacetic acid cauterization. Acta Otolaryngol 2003; 123:879–882. 47. Kim KH, Sung MW, Koh TY, et al. Pyriform sinus fistula: management with chemocauterization of the internal opening. Ann Otol Rhinol Laryngol 2000; 109:452–456. 48. Pereira KD, Smith SL. Endoscopic chemical cautery of piriform sinus tracts: a safe new technique. Int J Pediatr Otorhinolaryngol 2008; 72:185–188. 49. Cigliano B, Cipolletta L, Baltogiannis N, et al. Endoscopic fibrin sealing of congenital pyriform sinus fistula. Surg Endosc 2004; 18:554–556. 50. Leboulanger N, Ruellan K, Nevoux J, et al. Neonatal vs delayedonset fourth branchial pouch anomalies: therapeutic implications. Arch Otolaryngol Head Neck Surg 2010; 136:885–890. 51. Roh JL, Sung MW, Hyun Kim K, Il Park C. Treatment of branchial cleft cyst with intracystic injection of OK-432. Acta Otolaryngol 2006; 126:510–514. 52. Kim MG, Lee NH, Ban JH, et al. Sclerotherapy of branchial cleft cysts using OK-432. Otolaryngol Head Neck Surg 2009; 141:329– 334. 53. Nixon PP, Healey AE. Treatment of a branchial sinus tract by sclerotherapy. Dentomaxillofac Radiol 2011; 40:130–132. CHART 1 14 12 10 8 6 4 2 0 NO OF CASES FIGURE 1 (Cyst & Fistula) FIGURE 2 FIGURE 3 FIGURE 4