Module 4 – Safety and Health
advertisement

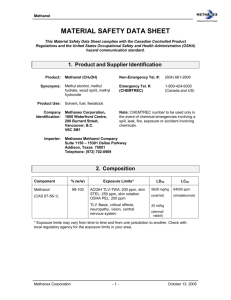
Module 4 – Safety and Health Learning Objectives After completing this Module, you should: 1. Be able to identify the appropirate personal protective equipment to be used during biodiesel production. 2. Be able to correctly operate an eye wash station. 3. Understand the major hazards posed by chemicals used in biodiesel production. 4. Understand the appropriate safety precautions required for chemicals used in biodiesel production. Introduction Biodiesel production involves materials and conditions that could cause serious injury, illness, or death if mishandled. It is critical that you understand and follow health and safety precautions. Serious breech of health or safety precautions can require a student's removal from the course. Personal Protective Equipment (PPE) Anytime you are involved in biodiesel production, you should be wearing the following PPE (available for you in the lab): Safety glasses Nitrile gloves Lab coat or tyvek coveralls Closed-toed shoes or booties Loading methanol and catalyst into the BioPro 190 requires a respirator. Since you have not been fitted with a respirator, you should not undertake these operations. Eye Wash Station Eye contact with methanol, KOH, sulfuric acid, or muriatic acid requires immediate use of the eye wash station. The biodiesel reactor sink is equiped with a handheld eye wash station and the agriculture lab has a fixed eye wash station. To use the eye wash, do the following Depress the lever or button to begin the flow of water. Do not remove the plastic caps covering the eye wash nozzles, as they will swing away once the flow of water starts. Lower your head into the basin until the jets of water come directly into contact with both eyes. Hold your eyes wide open using your thumbs and index fingers. Keeping the head steady, move your eyes around by looking from side to side and up and down. This will help rinse as much of the eye as possible. Continue rinsing for at least 15 minutes. If eye pain continues, seek medical care immediately. Methanol - Fire, Toxicity Flammability Methanol has a flash point of 12 deg C (53.60 deg F) and is very volatile. Therefore, the liquid as well as the vapors produce a fire hazard and the following precautions should always be followed: Do not handle in areas that have potential ignition sources - open flame, spark or hot surface. Do not handle or use methanol if welding operations are occuring in Ropp 108. Make sure containers are grounded (place container on floor) when pumping methanol to reduce the likelihood of static electricity accumulation. Keep containers closed except while transfering methanol. Always store methanol in the Flammables Cabinet and keep the cabinet doors closed when not in use. Check availability of a fire extinguisher before handling methanol. Note that in a methanol fire, the flame may not be visible. Toxicity Signficant explosure to methanol can cause serious illness or death. Below is the health warning from the material safety data sheet (MSDS) for methanol: Routes of Entry: Skin Contact: Moderate Eye Contact: Moderate Ingestion: Major Inhalation: Major Effects of Short-Term (Acute) Exposure: Inhalation: Inhalation of high airborne concentrations can also irriate mucous membranes, cause headaches, sleepiness, nausea, confusion, loss of consciousness, digestive and visual disturbances and even death. NOTE: Odour threshhold of methanol is several times higher than the TLV-TWA. Depending upon severity of poisoning and the promptness of treatment, survivors may recover completely or may have permanent blindness, vision disturbances and/or nervous system effects. Concentrations in air exceeding 1000 ppm may cause irritation of the mucous membranes. Skin Contact: Methanol is moderately irritating to the skin. Methanol can be absorbed through the skin and harmful effects have been reported by this route of entry. Effects are simialr to those described in "Inhalation" Eye Contact: Methanol is a mild to moderate eye irritant. High vapour concentration or liquid contact with eyes causes irritation, tearing and burning. Ingestion: Swallowing even small amounts of methanol could potentially cause blindness or death. Effects of sub lethal doses may be nausea, headache, abdominal pain, vomiting and visual disturbances ranging from blurred vision to light sensitivity. Effects of Long-Term (Chronic) Exposure: Repeated exposure by inhalation or absorption may cause systemic poisoning, brain disorders, impaired vision and blindness. Inhalation may worsen conditions such as emphysema or bronchitis. Repeated skin contact may cause dermal irritation, dryness and cracking. Medical Conditions Aggravated By Exposure: Emphysema or bronchitis. If handled according to our biodiesel production procedures, methanol will not pose a health hazard during biodiesel production. Take the following precautions: Wear appropirate PPE (glasses, gloves, suit). A respirator is not required unless loading into the reactor. Keep methanol out of your breathing zone. Wash skin and remove clothes that become contaminated with methanol. Quiz Me… Methanol is highly flammable and therefore must be… a. Placed in a container on the ground to reduce static electricity. b. Handled without possible ignition sources and therefore mustn't be handled during welding sessions at Ropp hall. c. Stored in a flammables cabinet. d. All of the above. Swallowing even small amounts of methanol can result in... a. Blindness b. Nausea c. Death d. All of the above. Potassium Hydroxide Catalyst (KOH) - Skin Burns, Toxicity KOH is highly caustic and will burn tissues on contact, espcially eyes. However, KOH is nonvolatile, so the exposure routes of greatest concern are direct contact with skin or eyes, and inhalation of the dust. Below are the health hazard warnings from the MSDS for KOH Eye: Causes severe eye burns. May cause irreversible eye injury. Contact may cause ulceration of the conjunctiva and cornea. Eye damage may be delayed. Skin: Causes skin burns. May cause deep, penetrating ulcers of the skin. Ingestion: Harmful if swallowed. May cause circulatory system failure. May cause perforation of the digestive tract. Causes severe digestive tract burns with abdominal pain, vomiting, and possible death. Inhalation: Harmful if inhaled. Irritation may lead to chemical pneumonitis and pulmonary edema. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. Chronic: Prolonged or repeated skin contact may cause dermatitis. Prolonged or repeated eye contact may cause conjunctivitis. The following precautions should always be followed: Wear appropirate PPE (glasses, gloves, suit). Respirator not required unless loading reactor. Handle KOH under a chemical hood. Avoid creating airborne KOH dust. Seal KOH containers for transport. Keep KOH away from acids and water. It can react violently. Wash skin and remove clothing that becomes contaminated with KOH. Use eye wash if contact with eyes occurs. Quiz me… KOH will not form a vapor, but inhalation of KOH dust can cause respiratory damage. True False All of the following are necessary precautions when working with KOH except one. Which is it? a. Handle KOH under a chemical hood. b. Always wear a respirator c. Gloves, safety glasses, and tyvek suit should be worn. d. Keep KOH away from acids. e. Wash skin if KOH contact occurs. Sulfuric Acid (H2SO4) - Skin Burns, Toxicity Sulfuric Acid is highly corrosive and will burn tissues on contact, espcially eyes. However, sulfuric acid is relatively non-volatile, so the exposure routes of greatest concern are direct contact with skin or eyes. Below are the health hazard warnings from the MSDS for sulfuric acid. Eye: Immediate pain, severe burns and corneal damage, which may result in permanent blindness. Skin: Causes burns, and brownish or yellow stains. Concentrated solutions may cause second or third degree burns with severe necrosis. Prolonged and repeated exposure to dilute solutions may cause irritation, redness, pain and drying and cracking of the skin. Inhalation: Causes respiratory irritation and at high concentrations may cause severe injury, burns, or death. Effects of exposure may be delayed. Ingestion: Causes severe irritation or burns of the mouth, throat, and esophagus. The following precautions should always be followed: Wear appropirate PPE (glasses, gloves, suit). Respirator not required even when loading reactor. Handle sulfuric acid under a chemical hood. Seal surfuric acid container for transport. Keep surfuric acid away from KOH and water. It can react violently. Wash skin and remove clothing that becomes contaminated with sulfuric acid. Use eye wash if contact with eyes occurs. Muriatic Acid (HCl, hydrochloric acid) - Skin Burns, Inhalation Toxicity Muriatic Acid is highly corrosive and will burn tissues on contact, espcially eyes. In addition, muriatic acid is volatile and represents a serious inhalation hazard. Below are the health hazard warnings from the MSDS for muriatic acid. Eye: Contact rapidly causes severe irritation of the eyes and eyelids. If not quickly removed by thorough irrigation with water, there may be prolonged or permanent visual impairment or total loss of sight. Hydrogen chloride gas escaping from the aqueous solution is immediately irritating. Skin: Contact may cause burns and tissue destruction. Inhalation: Breathing gas, fog, mist or spray may result in coughing and burning or choking sensation in the throat. If inhaled deeply, fluid may collect in the lungs (edema). Prolonged or repeated exposure to concentrations in excess of the exposure limits may cause discoloration of teeth. Ingestion: Can cause severe burns to the mucous membranes of the digestive tract. The following precautions should always be followed: Wear appropirate PPE (glasses, gloves, suit). Respirator not required even when adding to wash water. Handle under a chemical hood. Seal acid container for transport. Keep acid away from KOH and water. It can react violently. Wash skin and remove clothing that becomes contaminated with acid. Use eye wash if contact with eyes occurs. Quiz Me… Unlike sufuric acid, muriatic acid is volatile and posses a serious inhalation hazard. True False Unlike sulfuric acid, muriatic acid will not react violently with KOH. True False