Salinity vs. Density of Sea Water Purpose
advertisement
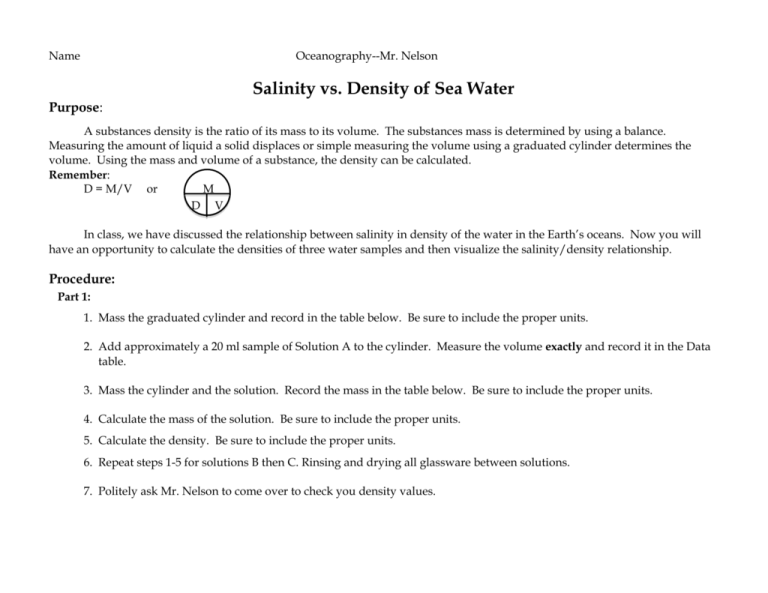
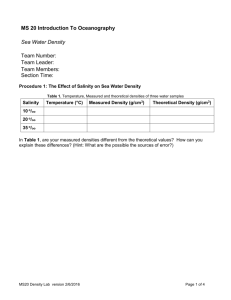
Name Oceanography--Mr. Nelson Salinity vs. Density of Sea Water Purpose: A substances density is the ratio of its mass to its volume. The substances mass is determined by using a balance. Measuring the amount of liquid a solid displaces or simple measuring the volume using a graduated cylinder determines the volume. Using the mass and volume of a substance, the density can be calculated. Remember: D = M/V or M D V In class, we have discussed the relationship between salinity in density of the water in the Earth’s oceans. Now you will have an opportunity to calculate the densities of three water samples and then visualize the salinity/density relationship. Procedure: Part 1: 1. Mass the graduated cylinder and record in the table below. Be sure to include the proper units. 2. Add approximately a 20 ml sample of Solution A to the cylinder. Measure the volume exactly and record it in the Data table. 3. Mass the cylinder and the solution. Record the mass in the table below. Be sure to include the proper units. 4. Calculate the mass of the solution. Be sure to include the proper units. 5. Calculate the density. Be sure to include the proper units. 6. Repeat steps 1-5 for solutions B then C. Rinsing and drying all glassware between solutions. 7. Politely ask Mr. Nelson to come over to check you density values. DATA TABLE: DENSITY OF THREE LIQUIDS SOLUTION A Mass of the cylinder AND solution SOLUTION B SOLUTION C FRESH WATER n/a Mass of cylinder n/a Mass of solution n/a Volume of solution n/a Density of solution 1.0 g/cm3 Salinity of solution 0 PPT Part 2: 1. Obtain a test tube. 2. Using your density figures from Part 1, a pipette, and the hint below, perfectly layer the three colored liquids. Hint: You will need to add the least dense liquid first. Then, carefully add the next densest liquid to the bottom of the test tube. Finally, you will carefully add the most dense liquid to the bottom of the test tube. If you are careful and don’t add the liquids hastily, you will get a nice density stratification. Air bubbles are your enemy! 3. Upon completing this density stratification, to his high Natick standards, show Mr. Nelson your test tube. He will type a secret code in the box below to indicate that you have successfully completed step #2. U S A !!!!!!!! Questions: RED FONT PLEASE 1. Find out the salinity of each solution, from Mr. Nelson, and record it in your data chart. 2. Research how to create a “best fit” line graph using Microsoft Excel. 3. Create this best fit line graph using Salinity (x-axis) and Density (y-axis). 4. Please plot the following four points: The salinity/density numbers for each of the three solutions and fresh water. Format your y-axis to start a 1.0 g/cm3. Attach your graph to the last page. 5. According to your graph, what is the relationship between Salinity and Density? 5a. Use the Essentials of Oceanography text or any other source. According to your source, what is the relationship between Salinity and Density in the ocean? 6. Based on #3 and #3a, use a clear sentence to state the relationship between Salinity and Density in seawater? 7. Do fish that live towards the bottom of the deep ocean have to be adapted to higher or lower levels of salinity, compared to fish that live near the surface. Why? Attach your graph here.