Ocean Chemistry and Composition
advertisement

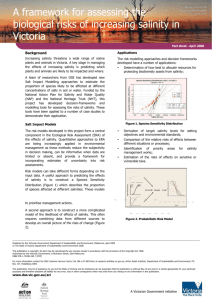
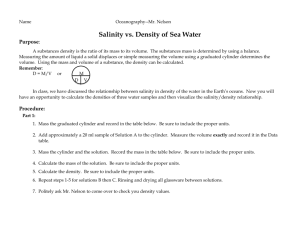
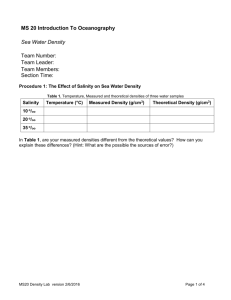
Ocean Chemistry and Composition Brian Schuster Chemical Properties of Sea Water • polar: unequal sharing of electrons • hydrogen bonding: intermolecular dipole-dipole interaction between H2O molecules • cohesion: sticking of H2O molecules to each other resulting from hydrogen bonding; causes surface tension • adhesion: sticking of H2O molecules to other polar materials • capillarity: movement of water up a small tube • latent heat: energy stored in water that doesn’t change it’s temperature Acidity/Alkalinity • • • ocean: pH 7.5 to 8.5 due to carbonate (CO3-2) from dissociation of calcium carbonate freshwater: pH 6.5 to 7.5 due to carbonic acid (H2CO3) bicarbonate buffering, resistance to pH change – – – H2O + CO2 H2CO3 (carbonic acid=weak acid) H2CO3 H+ + HCO3- (bicarbonate=buffer) CaCO3 Ca+2 + CO3-2 (carbonate=base) Temperature • depth dependent • thermocline: rapid change in temperature around a certain depth; more defined near equator; less prominent at poles • isotherm: line of constant temperature Global isotherms display currents Salinity • isohaline: line of constant salinity • residence time: average time a molecule spends in a certain reservoir • salinometers: determine salinity through conductivity • halocline: rapid change in salinity • constancy of composition: major ion constituents exist in constant proportions • water is the “universal solvent” • ocean salinity: 35ppt, brackish: 17ppt, brine: >50ppt • saltiest sea: Dead Sea Salinity (continued) • Long-term sources: – hydrothermal vents & volcanoes – weathering of rocks • cation: positively charged ion • anion: negatively charged ion • salinity effected by: – precipitation, evaporation – freezing, thawing – river input • Top six constituents – – – – – – chlorine (Cl-) 55% sodium (Na+) 31% sulfate (SO4-2) 8% magnesium (Mg+2) 4% calcium (Ca+2) 1% potassium (K+) 1% Desalination 1. 2. 3. 4. reverse osmosis freezing & thawing ion exchange distillation (evaporation & condensation) Density • pycnocline: rapid change in density • freshwater most dense at 4 °C, saltwater most dense just before freezing • saltwater freezes at -2 °C (freezing point depression) • density affected by: – temperature – salinity – depth, pressure: minimally • isopycnal: surface of constant density Pressure and Gases • rises by 1 atm (14.7 psi, 101.3 kPa) for every 10 m (33 ft) depth • absolute pressure at 20 m is 3 atm • gauge pressure at 20 m is 2 atm • isobar: line of constant pressure Dissolved Gases • Henry’s Law: more gas can be dissolved under high pressure • more gas is dissolved in deep, cold water • oxygen minimum zone: depth depends on productivity and aerobic respiration • atmospheric gases resemble ocean’s due to mixing Calcium Carbonate (CaCO3) • used in shells, compound of limestone and calcite, base of coral reef • sources and sinks – chemical precipitation, dissolution – weathering of limestone and calcite – organisms • solubility increased by: – greater depths – higher acidity (lower pH) • carbonate compensation depth (CCD): dissolution rate of CaCO3 equals supply rate Nutrients • eutrophication: excessive addition of nutrients • oligotrophication: excessive removal of nutrients • iron is the limiting nutrient for most algae South Atlantic phytoplankton bloom Heat Transfer • conduction: molecules speed each other up by physically bumping • convection: carried by movement of fluid • radiation: electromagnetic radiation is absorbed and raises temperature • heat is conducted faster in water than in air, so divers can get cold faster Sound • in water, speed of sound = ~1500 m/s (3500 mph), 5x speed in air • speed changes with density • sound travels farther in water than light, so it is good for cetacean communication • SOFAR (sound fixing and ranging) channel: sound travels slower around 1000 m, and can go farther; sound gets stuck in this channel Light • penetration – long wavelengths (red) absorbed first – mid-range wavelengths (green, blue) go farthest • attenuation: decrease in light intensity due to absorption and scattering by suspended particles • turbidity increases attenuation • index of refraction (n) = 1.33 Other • conservative property: mostly affected by mixing and diffusion (ex: salinity) • non-conservative property: affected mostly by processes other than mixing and diffusion (ex: dissolved oxygen relating to productivity) • temperature-salinity diagram: unique to different bodies of water; shows lines of constant density in sigma units