Explaining and how to calculate the relative atomic mass RAM or Ar
advertisement
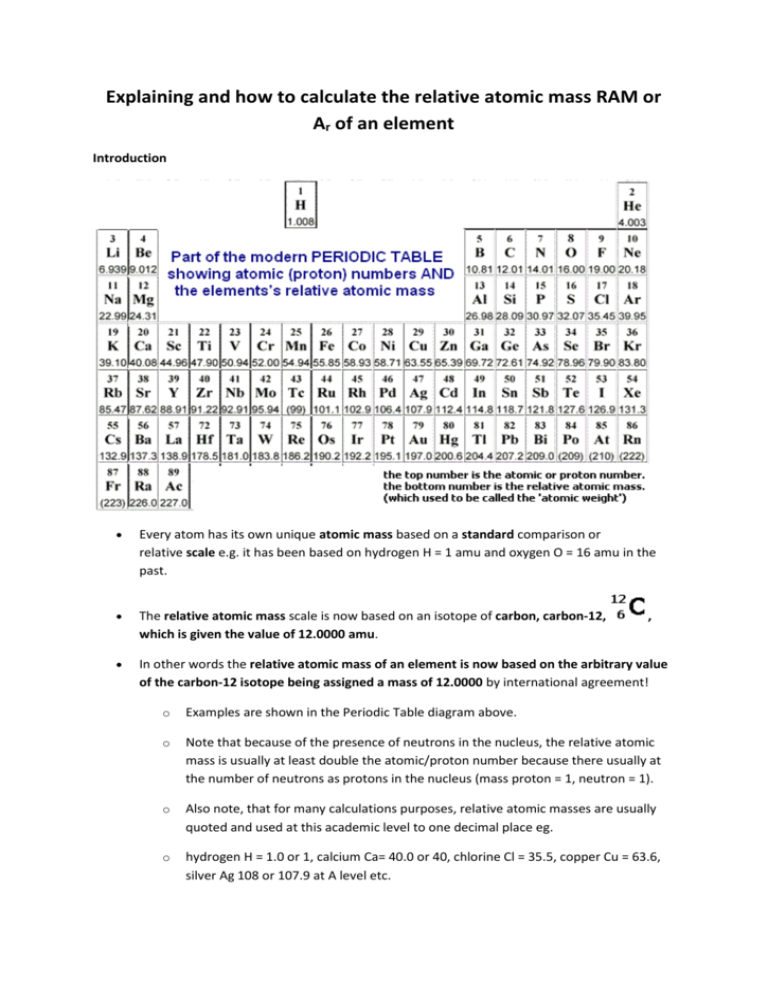
Explaining and how to calculate the relative atomic mass RAM or Ar of an element Introduction Every atom has its own unique atomic mass based on a standard comparison or relative scale e.g. it has been based on hydrogen H = 1 amu and oxygen O = 16 amu in the past. The relative atomic mass scale is now based on an isotope of carbon, carbon-12, which is given the value of 12.0000 amu. In other words the relative atomic mass of an element is now based on the arbitrary value of the carbon-12 isotope being assigned a mass of 12.0000 by international agreement! , o Examples are shown in the Periodic Table diagram above. o Note that because of the presence of neutrons in the nucleus, the relative atomic mass is usually at least double the atomic/proton number because there usually at the number of neutrons as protons in the nucleus (mass proton = 1, neutron = 1). o Also note, that for many calculations purposes, relative atomic masses are usually quoted and used at this academic level to one decimal place eg. o hydrogen H = 1.0 or 1, calcium Ca= 40.0 or 40, chlorine Cl = 35.5, copper Cu = 63.6, silver Ag 108 or 107.9 at A level etc. o Sometimes at A level, values of relative atomic masses to two decimal places may be quoted. In using the symbol Ar for RAM you should bear in mind that the letter A on its own usually means the mass number of a particular isotope and amu is the acronym shorthand for atomic mass units) However there are complications due to isotopes and so very accurate atomic masses are not whole numbers. Isotopes are atoms of the same element with different masses due to different numbers of neutrons. The very accurate atomic mass scale is based on a specific isotope of carbon, carbon-12, 12C = 12.0000 units exactly, for most purposes C = 12 is used for simplicity. For example , and are the three isotopes of hydrogen, though the vast majority of hydrogen atoms have a mass of 1. When their accurate isotopic masses, and their % abundance are taken into account the average accurate relative mass for hydrogen = 1.008, but for most purposes H = 1 is good enough! The strict definition of relative atomic mass (Ar) is that it equals average mass of all the isotopic atoms present in the element compared to 1/12th the mass of a carbon-12 atom. o So, you are taking into account the different isotopic masses of the same elements, but also their % abundance in the element. o Therefore you need to know the percentage (%) of each isotope of an element in order to accurately calculate the element's relative atomic mass. Examples of relative atomic mass calculations for GCSE/IGCSE/AS level students and Example 1.1: bromine consists of 50% 79Br and 50% 81Br, calculate the Ar of bromine. o Ar = [ (50 x 79) + (50 x 81) ] /100 = 80 o So the relative atomic mass of bromine is 80 or RAM or Ar(Br) = 80 o Note the full working shown. Yes, ok, you can do it in your head BUT many students ignore the %'s and just average all the isotopic masses (mass numbers) given, in this case bromine-79 and bromine-81. and Example 1.2: chlorine consists of 75% chlorine-35 and 25% chlorine-37. o Think of the data based on 100 atoms, so 75 have a mass of 35 and 25 atoms have a mass of 37. o The average mass = [ (75 x 35) + (25 x 37) ] / 100 = 35.5 o So the relative atomic mass of chlorine is 35.5 or RAM or Ar(Cl) = 35.5 o Note: 35Cl and 37Cl are the most common isotopes of chlorine, but, there are tiny percentages of other chlorine isotopes which are usually ignored at GCSE/IGCSE and Advanced GCE AS/A2 A level. Example 1.3: The mass number for any isotope is the sum of the protons and neutrons in the nucleus, and is always an integer i.e. a whole number. Examples for Advanced Level Chemistry students only (a) Calculation of relative atomic mass Relative isotopic mass = the accurate mass of a single isotope of an element compared to 1/12th the mass of a carbon-12 atom e.g. the accurate mass of is58.9332 ! If we were to redo the chlorine example 1.1 above, which is quite adequate for GCSE purposes, more accurately at A level, we would do .... chlorine is 75.77% 35Cl of isotopic mass 34.9689 and 24.23% 37Cl of isotopic mass 36.9658 so Ar(Cl) = [(75.77 x 34.9689) + (24.23 x 36.9658)] / 100 = 35.4527 (but 35.5 is usually ok in calculations pre-university!) See also Mass Spectrometer and isotope analysis on the GCSE-AS(basic) Atomic Structure Notes, with further RAM calculations. (b) Calculations of % composition of isotopes It is possible to do the reverse of a relative atomic mass calculation if you know the Ar and which isotopes are present. It involves a little bit of arithmetical algebra. The Ar of boron is 10.81 and consists of only two isotopes, boron-10 and boron-11 The relative atomic mass of boron was obtained accurately in the past and mass spectrometers can sort out the isotopes present. If you let X = % of boron 10, then 100-X is equal to % of boron-11 Therefore Ar(B) = (X x 10) + [(100-X) x 11) / 100 = 10.81 so, 10X -11X +1100 =100 x 10.81 -X + 1100 = 1081, 1100 - 1081 = X (change sides change sign!) therefore X = 19 so naturally occurring boron consists of 19% 10B and 81% 11B (the data books quote 18.7 and 81.3)