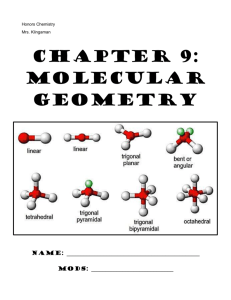
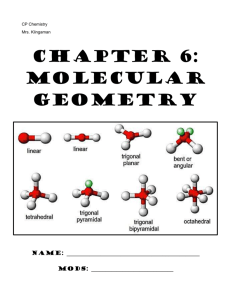
Ch. 9: Molecular Geometry & VSEPR Theory
advertisement

Ch. 9: Molecular Geometry & VSEPR Theory 1. VSEPR theory enables us to predict the shape (molecular geometry) of molecules based on the representative Lewis structure for each molecule. VSEPR stands for: V- _________________________ P- _________________________ S- _________________________ R- _________________________ E- _________________________ 2. VSEPR theory predicts the _______ ______________ of molecules based on the fact that there is ______________________ between the valence electrons surrounding the central atom of molecule. a. Bonding electrons pairs (single/double/triple bonds) take up space in a molecule and repel one another b. Nonbonding electron pairs also take up ___________ space in a molecule and repel electrons ___________ strongly than bonding electron pairs c. As a result of this repulsion, the atoms in a molecule space themselves out ______ __________ _________________ from one another as possible. VSEPR “The best arrangement of a given number of electron domains is the one that minimizes the repulsions among them.” 3. Fill in the table below: Name & Formula Lewis structure # atoms bonded to central # nonbonding electron pairs around central # of domains around central ABN type* Molecular Shape Polar (P) or Nonpolar (NP) Methane CH4 Ammonia NH3 Water H2O * Write the ABN formula for each of these molecules. A= central atom, B= number of attached atoms, N= Number of nonbonding electron pairs. The VSEPR chart (found in your reference sheets) lists possible shapes of molecules, based on their Lewis structure and ABN type. Be comfortable with interpreting this information…like you just did! The website below is excellent for visualizing the 3D orientation of each different molecular geometry or for building example molecules Online Simulator: http://phet.colorado.edu/en/simulation/molecule-shapes 4. Polarity: Polarity: physical property of a substance which results from an uneven distribution of positive and negative charge in a molecule or chemical bond Polar Bonds: We know (from chapter 8) that polar bonds result from having a significant difference in the _____________________________ of the two bonding atoms Molecular Polarity: When it comes to an entire molecule being polar, it must contain polar bonds and be arranged in space 3-dimensionally so that there is an uneven distribution of positive and negative charge (ie: the molecular geometry is important!!!) a. This results in one portion of the bond being partially negative (ϭ-) and the other half being partially positive (ϭ+) A molecule could contain polar bonds without the entire molecule being polar Polar Molecules: A polar molecule must contain polar bonds and have those polar bonds arranged in space 3-dimensionally (aka: the molecular geometry) so that one side of the molecule is partially negative (ϭ-) and the other side is partially positive (ϭ+) These molecules are considered to be ____________________________ in shape (most often due to the presence of _______________________ electron pairs on the central atom). Polar molecules form opposite “poles” within the molecule: one positive, one negative b. Nonpolar Molecules: These molecules are considered to be very __________________________ in shape (“no poles”). Either these molecules contain NO polar bonds, or any polar bonds it does contain are “canceled” out by the 3D shaping of the molecule Now You Try: Molecule ClO3– CHO– Lewis structure # atoms bonded to central # nonbonding electron pairs around central # of domains around central ABN type* Molecular Shape Polar (P) or Nonpolar (NP) VSEPR – Predicting Molecular Shapes Directions: Fill in the table below by drawing the Lewis Structure of the molecule and determining its ABN type and molecular shape Molecule CCl4 CO2 ClF3 XeBr2 Lewis structure # atoms bonded to central # nonbonding electron pairs around central # of domains around central ABN type* Molecular Shape Polar (P) or Nonpolar (NP) Molecule IBr5 PH5 NO2– XeF4 Lewis structure # atoms bonded to central # nonbonding electron pairs around central # of domains around central ABN type* Molecular Shape Polar (P) or Nonpolar (NP) Molecule HCN CHBr3 HCl O3 I3– Lewis structure # atoms bonded to central # nonbonding electron pairs around central # of domains around central ABN type* Molecular Shape Polar (P) or Nonpolar (NP)