Chapter 6: Molecular Geometry
advertisement
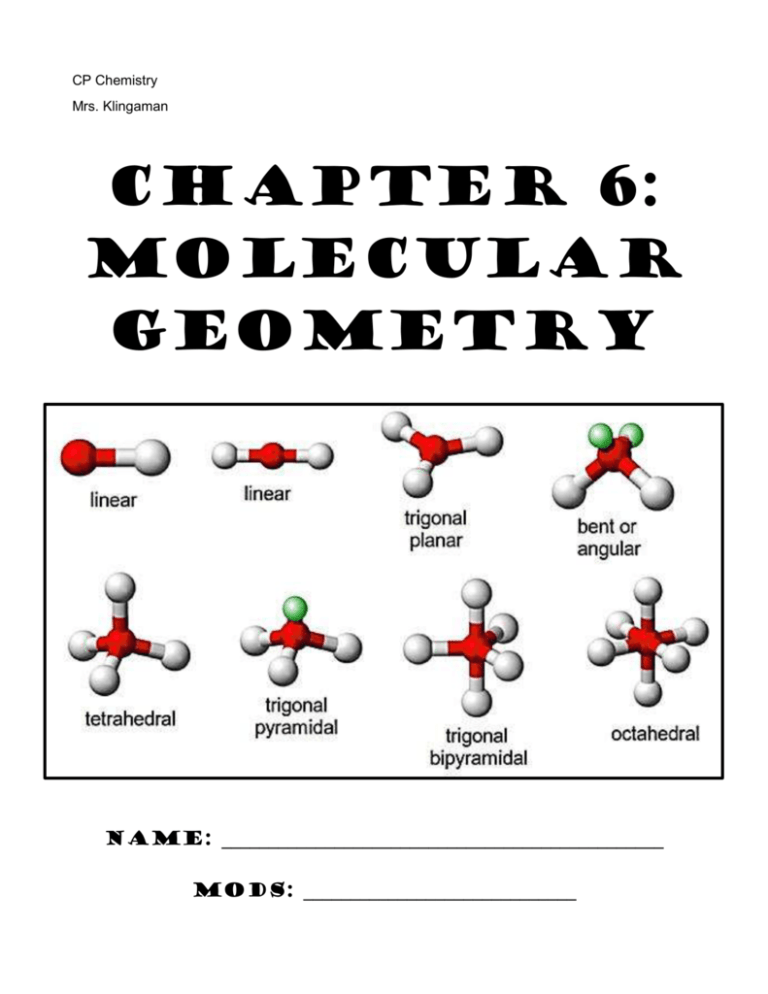
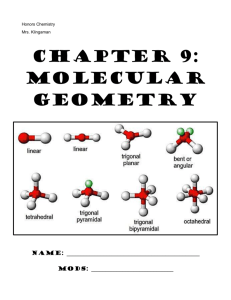
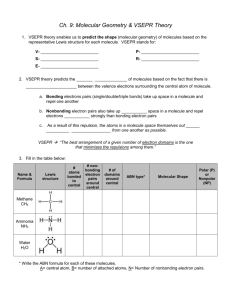
CP Chemistry Mrs. Klingaman Chapter 6: Molecular GEOMETRY Name: _______________________________________________ Mods: _____________________________ Chapter 6: Molecular Geometry 1. VSEPR theory enables us to predict the shape (molecular geometry) of molecules based on the representative Lewis structure for each molecule. VSEPR stands for: V- ___________________________ S- ___________________________ E- ___________________________ P- ___________________________ R- ___________________________ 2. VSEPR theory predicts the _________________________ of molecules based on the fact that there is ____________________________ between the valence electrons surrounding the central atom of molecule. a. Bonding electrons pairs (single/double/triple bonds) take up space in a molecule and repel one another b. Unshared/nonbonding electron pairs also take up space in a molecule and repel electrons __________________ strongly than bonding electron pairs c. As a result of this repulsion, the atoms in a molecule space themselves out ____________________________________ from one another as possible. *** 3. Fill in the table below: Name & Formula Lewis structure # atoms bonded to central # nonbonding electron pairs around central # of domains around central ABN type* Molecular Shape Methane CH4 Ammonia NH3 Water H2O * Write the ABN formula for each of these molecules. A= central atom, B= number of attached atoms, N= Number of lone pair electrons. Use this notation to determine the shape of each molecule and fill in your answer in the table. 4. Molecular Polarity: Understanding molecular shape allows us to think about molecular polarity. Molecules that are symmetrical are often considered non-polar Molecules that are asymmetrical (not the same on all sides) are often considered polar. Using the Lewis structures and molecular shape from the table in #3 above, predict if the molecules below are polar or nonpolar: a) Methane = __________________________ b) Ammonia = _________________________ c) Water = ___________________________ Now You Try: Molecule Lewis Dot Structure # of Atoms Bonded to Central # of Nonbonding e– Pairs # of domains around central ABN type Molecular Shape ClO3– CHO– Based on molecular shape and symmetry, circle the correct polarity of each molecule: ClO3– : polar or nonpolar CHO– : polar or nonpolar The chart at the front of this packet lists possible shapes of molecules, based on their Lewis structure and ABN type. Be comfortable with interpreting this information…like you just did! VSEPR – Predicting Molecular Shapes Directions: Fill in the table below by drawing the Lewis Structure of the molecule and determining its ABN type and molecular shape Molecule CCl4 CO2 ClF3 XeBr2 Lewis Dot Structure # of atoms bonded to central # of nonbonding e– pairs on central # of domains around central ABN Type Molecular Shape Molecule IBr5 PH5 NO2– SCl6 XeF4 Lewis Dot Structure # of atoms bonded to central # of nonbonding e– pairs on central # of domains around central ABN Type Molecular Shape Molecule HCN CHBr3 HCl O3 I3– Lewis Dot Structure # of atoms bonded to central # of nonbonding e– pairs on central # of domains around central ABN Type Molecular Shape