Medical Necessity for Cough Assist
advertisement

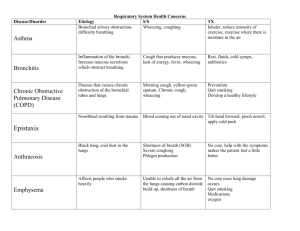
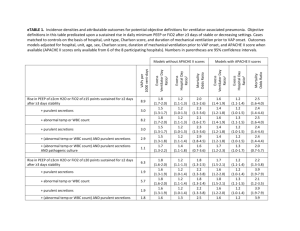
Draft of letter of Medical Necessity for Cough Assist (In-Exsufflator) From Dr. Bach (www.doctorbach.com). Medical Necessity for CoughAssist™ Cough Stimulating Device for person with neuromuscular disease (NMD) or high-level spinal cord injury (SCI) There are many medications and medical devices that assist a patient in loosening bronchial secretions for easier removal. These medications and devices are useful for patients suffering from cystic fibrosis, chronic bronchitis, pneumonia, and other conditions where an excess of thick secretions build up within the lungs. Once loosened, the bronchial secretions are then removed from the lungs through a "productive cough" which moves the secretions up the trachea and out the mouth. A cough is produced when the diaphragm and chest muscles work to expand the lungs (full inhalation) and then rapidly contract the lungs to expel the air (explosive exhalation), and along with the air, the loosened secretions. Patients with neuromuscular disease (NMD) or high-level spinal cord injury (SCI) often lack the muscle strength (NMD) or muscle control (SCI) to create the full inhalation/explosive exhalation required to cough and thereby remove bronchial secretions from the lungs. Without removal of these secretions bacterial infection (pneumonia) and oxyhemoglobin desaturation (low blood oxygen) can occur resulting in respiratory failure, hospitalization, and death. The situation is exacerbated for patients with poor bulbar (swallowing) muscle control as they may also "aspirate" saliva, foods, or liquids into the lungs. If the aspirated material is not removed, bacterial infection (pneumonia) and oxyhemoglobin desaturation (low blood oxygen) can occur resulting in respiratory failure, hospitalization, and death. For patients with a tracheostomy (breathing tube inserted into the trachea through the neck), it is common practice to utilize a suctioning device for removal of bronchial secretions. A small "vacuum cleaner" hose is inserted directly into the lungs through the tracheostomy opening (or through the attached ventilation hoses) to suction out the secretions. For patients without a tracheostomy, the only device of which we are aware that provides the full inhalation/explosive exhalation required for clearing the lungs of bronchial secretions and aspirated matter is the CoughAssist™ (J H Emerson Co, Cambridge, Massachusetts), formerly known as the In-Exsufflator, based on the Cofflator from O.E.M. Corporation. The CoughAssist™ is distributed by Respironics, Inc. The CoughAssist™ is a portable electrically powered device that utilizes a blower and a valve to alternately apply positive then negative pressure to the patient's airway in order to assist the patient in clearing retained bronchial secretions. Air is delivered to/from the patient via a breathing circuit incorporating a flexible tube, a bacterial filter, and either a facemask or mouthpiece. Cough Stimulating Device Medicare HCPCS Code E0482 E0482 - Cough stimulating device, alternating positive and negative airway pressure Effective January 1, 2002 Formerly covered under E1399 Medical necessity documentation is required Coverage: 15 months capped rental Medicare Fee Schedule reimbursement range: $363.71 to $427.89 After 15-month rental cap, maintenance and servicing fees may be billed once every 6 months. To the best of our knowledge there is only one product covered by this code: CoughAssist™ (J H Emerson Co, Cambridge, Massachusetts), formerly known as the In-Exsufflator, and distributed by Respironics, Inc. The CoughAssist™, based on the Cofflator from O.E.M. Corporation used during the polio epidemics in the 1950s, is a proven technology that fell into disuse in the early '60s after tracheostomy and suctioning became more popular. It has regained acceptance as a noninvasive alternative for clearing airway secretions. By avoiding invasive surgery (tracheotomy) when noninvasive alternatives are viable, both quality of life and mortality rates are improved as documented in Chest, Am J Phys Med Rehabil, Arch Phys Med Rehabil, Rehabilitation R&D Progress Reports (Veterans Health Administration), Distrofia Muscolare, Bull N Y Acad Med, and other medical sources. Use of the CoughAssist™ is indicated for the management of retained secretions where the patient is unable to generate a sufficient unassisted cough flow rate to clear secretions (< 160 to 270 L/min). The adoption of a noninvasive protocol (the use of CoughAssist™ in conjunction with noninvasive positive pressure ventilation) has been shown to increase patient's quality of life, decrease respiratory-related hospitalization from 21 days/year to less than 2 days/year, and prolong life, in many cases by over 50 years. (Prevention of Pulmonary Morbidity). This patient suffers from ___ neuromuscular disease / high-level spinal cord injury. This patient has an insufficient cough flow rate to clear secretions (_____ L/min). This patient has diminished bulbar function that results in aspiration of saliva, food and/or liquids. There is an absolute medical necessity for this patient to have prompt access to a CoughAssist™ device for removal of bronchial secretions. There is an absolute medical necessity for this patient to have prompt access to a CoughAssist™ device for removal of aspirated matter. Failure to provide this patient with prompt access to a CoughAssist™ device will likely result in repeated oxyhemoglobin desaturation, bronchial infection, pulmonary failure, hospitalization, and death. The only viable alternative to the use of a CoughAssist™ device is invasive surgery to install a tracheostomy tube resulting in hospitalization, higher medical care and equipment costs, lower quality of life, and premature death. Contact Information: J.H. Emerson Company 22 Cottage Park Avenue Cambridge, Massachusetts 02140-1691 1-800-252-1414 www.CoughAssist.com www.JHEmerson.com Respironics, Inc. 1501 Ardmore Blvd. Pittsburgh, PA 15221-4401 1-800-638-8208 www.Respironics.com Prepared by: Rich Clingman, Webmaster www.DoctorBach.com Revised: April 16, 2002