Mole Concept & Molar Mass Notes: Chemistry Stoichiometry
advertisement

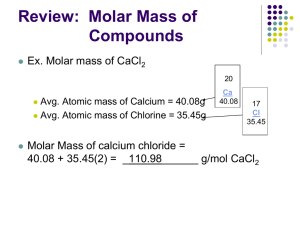
The Mole’s Unit NOTES Name_____________________________ Period_____ The Mole The mole (also abbreviated as mol) is the SI unit of measure for the amount of a substance. It is based on the number of representative particles in 12 g of C-12. A representative particle is any kind of particle, atoms, molecules, formula units, electrons or ions. The number 6.02 x 1023 is called Avogadro’s number. This is the number of particles in one mole. So if you had one mole of M&M’s that would be equal to 6.02 x 1023 M&M’s! 602,000,000,000,000,000,000,000.0 pieces of anything Molar Mass >The mass in grams of one mole of any pure substance is called its molar mass. >The molar mass of any element is equal to its atomic mass and has the unit of g/mol. *Remember, find atomic mass (average atomic mass) by looking elements up on the periodic table! Example: The molar mass of Cu is 63.54 g/mol one mole of Cu = 63.54 g The molar mass of Au is 196.967 g/mol one mole of Au = 196.967g The molar mass of He is 4.003 g/mol one mole of He = 4.003g Molar Mass of Compounds To determine the molar mass of a compound, you need to calculate the molar mass of each individual element in the compound, and then add them all together. The units for molar mass are always g/mol (grams / mole) *Remember that the molar mass of each element is its atomic mass on the periodic table. To find mass of each element in the compound Element 1 = (# of moles) x (molar mass) = mass in grams Element 2 = (# of moles) x (molar mass) = mass in grams Then you add up all of your answers. (Element 1 mass in g) + (Element 2 mass in g) = TOTAL Molar Mass for the Compound Examples: H2O (Water) Mass of H= (2 moles) x (1.008g) = 2.016g Mass of O= (1 mole) x (15.999) = 15.999g TOTAL Molar Mass of H2O = 2.016g + 15.999g = NOTES PAGE 1 18.015g/mol Find the molar mass of the following compound: Calcium Phosphide (Ca3P2) Mass of Ca= (3 moles) x (40.078g) = 120.234g Mass of P = (2 moles) x ( 30.974g) = 60.148g TOTAL Molar Mass of Ca3P2= 120.234 + 60.148g = 180.382g/mol Aluminum Sulfite [Al2(SO4)3] Mass of Al = (2 moles) x (26.982g) = 53.964g Mass of S = (3 moles) x (32.066g) = 96.198g Mass of 0 = (12 moles) x (15.999g) = 191.988g TOTAL Molar Mass of Al2(SO4)3 = 53.964g + 96.198g + 191.988g = 342.150 g/mol *Remember, you are still responsible for knowing how to write formulas of ionic compounds See your Ionic Compounds Unit Homework Packet for how to write formulas!! Questions can and will just give you the name of the compound and will require you to find the formula before you are able to calculate the molar mass (just like the previous examples). Put the following elements & compounds in order of INCREASING molar mass (Smallest) mass is… ___________H__________ Neon (Ne) _____________ __________Ne___________ Oxygen (O2) _____________ __________O2___________ Hydrogen (H2) _____________ ______Table Salt_________ Table Salt (NaCl) _____________ _________Acetate________ Copper (Cu) _____________ _________Copper_________ Acetate (C2H3O2) _____________ (Biggest) NOTES PAGE 2 Percent Composition When we talk about compounds it can be useful to know what elements are in the compound, and relatively how much of each element there is in the compound. We talk about “how much” of each element in terms of percentages. The percent composition then tells what percent of the whole compound is element 1, what percent of the whole compound is element 2, and what percent of the whole compound is element 3. If these 3 elements are the ONLY things in the compound, then the percentages should add up to 100%. To calculate the % Composition: % composition = (total mass of element/ mass of compound) x 100% **Usually you have to find the mass of each element, and the Total mass of the compound before you can calculate the % composition Let’s find the % composition of the elements in water H2O : Mass of H2 = 1.008 x 2 moles = 2.016g Mass of O = 15.999 x 1 mole = 15.999g Mass of H2O = 2.016g + 15.999g = 18.015 g/mol of H2O Mass of H2 = 2.016g % H = (2.016 /18.015) x 100% = 11.19 % H Mass of O = 15.999g % O = (15.999/18.015) x 100% = 88.81% O Total = 11.19% + 88.81% = 100% water Practice: Aluminum Oxide: find the % composition of Al and of O in this compound Find the formula: Al2O3 Mass of Al2: 26.982 x 2 moles = 53.964g Mass of O3: 15.999 x 3 moles = 47.997g Molar Mass of Al2O3: 53.964g + 47.997g = 101.961g/mol of Al2O3 % Al: (53.964/101.961) x 100% = 0.52926 x 100% = 52.93% % O: (47.997/101.961) x 100% = 0.4707 x 100% = 47.07% Total = 52.93% + 47.07%= 100% NOTES PAGE 3 Moles to Grams & Grams to Moles We are able to convert between number of moles and the mass (in grams) of a substance easily by using the molar mass of the atom or compound! We just use dimensional analysis to solve these! To convert moles of a compound to mass: simply multiply the number of moles of the compound by the molar mass of the compound. For example 8.55 moles of K2CrO4 would be calculated as follows: 2 mol K x 39.10 g/ mol = 78.20 g 1 mol Cr x 52.00 g/ mol = 52.00 g 4 mol O x 16.00 g/ mol = 64.00 g Molar mass K2CrO4 = 194.20 g/mol 8.55 mols of K2CrO4 x 194.20 g = 1660.41 g 1 1 mole Examples: 1.50 mol Potassium = __________________ g 0.750 mol Aluminum = __________________ g 3.850 mol Lithium Fluoride = __________________ g Going from mass to moles is the same process in reverse! It is still just dimensional analysis. Examples: 148 g Argon 78.3 g Rubidium Fluoride = __________________ mol = __________________ mol NOTES PAGE 4 Moles to Particles & Particles to Moles **Particle is generic way of saying atom, formula units, or molecule Different ways of saying the same thing: Atom: We say atom when it is just one pure element with no bonds (Ex. Al , Cu, C, Ne…1 element) Formula Unit: when we are talking about ionic compounds (metal bonded with nonmetals) Ex. NaCl Molecule: is for covalent compounds (two or more nonmetals bonded together) Ex. CO2 It’s nice and easy when we talk about 1 mole at a time. But sadly, not always the case. Let’s try thinking about eggs first, since they are easier to visualize and we are more comfortable with “a dozen eggs” than we are “a mole of atoms” It’s dimensional Analysis! If you have 1 dozen eggs you have = 12 eggs If you have 1.75 dozen eggs you have… 1.75 x 12 = 21 = 21 eggs Moles to Atoms Calculations If you have 1 mole of Copper you have = 6.02 x 1023 atoms of Cu If you have 1.75 moles of Copper you have = 1.0535 x 1024 atoms of Cu 1.75 x (6.02 x 1023) = (1.0535 x 1024)atoms of Cu It’s dimensional Analysis! (what you are given) (what you are looking for) “Disco” your units down Examples: 3.25 mol AgNO3 = __________________ formula units AgNO3 11.5 mol H2O = __________________ molecules H2O 0.4 mol of Au = ___________________ atoms Au NOTES PAGE 5 Going from particles to moles is the same process in reverse! But it’s still just dimensional analysis. If you have 12 eggs you have = 1 dozen If you have 27 eggs you have = 2.25 dozen 27 eggs / 12 per dozen = 2.25 dozen Atoms to Moles Calculations : If you have 6.02 x 1023 atoms of Lithium you have = 1 mol Li If you have 2.0468 x 1024 atoms of Lithium you have = 3.4 mol Li Examples: 2.58 x 1024 atoms of Calcium = __________________ mol Ca 3.6 x 1023 molecules of CH4 = __________________ mol CH4 2.04 x 1024 formula units of KBr = __________________ mol KBr Grams to Particles & Particles to Grams We are able to go directly from mass (in grams) to number of particles (atoms, formula units, or molecules) by using Avogadro’s number and the molar mass. Examples: 29 g Helium = __________________ atoms He 36.08 g CO2 (44.009 g is molar mass) = __________________ molecules CO2 0.50 g ZnS (97.386 is molar mass) = __________________ formula units ZnS Going from particles to mass is the same process in reverse! But it is still just dimensional analysis Examples: 1.98 x 1023 atoms Titanium = ________________ g Ti 8.500 x 1023 atoms Xe = ________________ g Xe 1.187 x 1024 molecules Cl2 = ________________ g Cl2 NOTES PAGE 6