Discussion Problem Set 6 (Lectures 15
advertisement

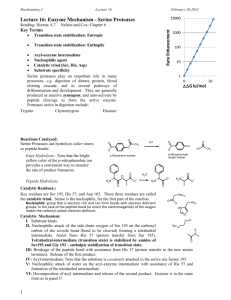
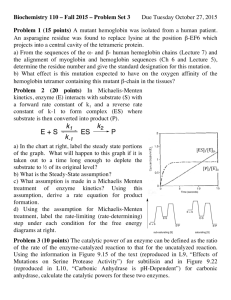
Discussion Problem Set 6 (Lectures 15-17) C483 N.B. These questions are designed to probe some mechanistic issues a bit more deeply. As such, they will be a bit more conceptually challenging than previous problem sets. Try to use them as thought problems and exercise your thinking a bit. 1. Serine proteases have been the target of inhibitor design for many years. Trifluoromethyl ketones (RCOCF3)are excellent inhibitors of such enzymes, much better than methyl ketones (RCOCH3). Explain why this might be so. 2. Dialkyl fluorophosphates are excellent irreversible inhibitors of serine proteases. Draw a mechanism for this inhibition. Why are such molecules better inhibitors than Acylfluorides? (give two reasons). 3. The kcat for hydrolysis of a series of ester substrates catalyzed by serine proteases, is usually about the same, regardless of the nature of the leaving group being cleaved. However, kcat usually varies for amide substrates and is substantially slower, changing even more as a function of leaving group. Explain this phenomenon and draw a free energy profile that accounts for this. 4. The enzyme lysozyme normally hydrolyzes beta1,4-linked NAM-NAG copolymers quite well. Evidence is that there is a carbohydrate binding site that binds up to six carbohydrate residues of the polymer. The enzyme also cleaves a polymer made up exclusively of NAG residues. (This is the polymer that chitin is made of. Chitin is the principal constituent of shellfish, insect and arthropod skeletons, and is undigestible by us, since we do not have lysozyme in our digestive tracts.) Residues bind in sites ABCDEF, with cleavage taking place between the 4th and 5th residues (D and E binding site). A NAG trisaccharide is a very good inhibitor, binding in the A,B, and C sites. However, a NAG tetrasaccharide binds more poorly than the trimer, in spite of the possibility of making more contacts to the enzyme. Explain this based on theories of enzyme catalysis. Why was the inhibition of a pentasaccharide of NAG not measured? 5. The book shows an older mechanism for lysozyme that is now thought to be incorrect. What is the new, accepted mechanism for this enzyme? On what basis did the new mechanism replace the old one? (You should look at the reading on the web site for some answers to this.) How does the new mechanism still fit with the evidence that supported the old one, such as the x-ray structure and model building, as well as the experiment described in question 4. 6. Draw the mechanism for TIM. A neutral His acts as an acid in this mechanism. Is this surprising? Explain what advantage might be gained by using such a mechanism. 7. What is the role of the carboxylate (Asp or Glu, depending on the enzyme) in the catalytic triad of serine proteases?