Rituximab in AIHA - Leeds South and East CCG
advertisement

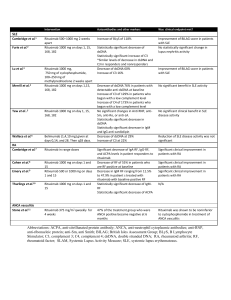
Commissioning statement 01/13 Treatment (brand name, manufacturer) For the treatment of Rituximab Commissioning position Rituximab is routinely commissioned by Leeds West CCG, Leeds North CCG and Leeds South and East CCG as an option for 2nd line treatment in patients with either warm or cold Autoimmune Haemolytic Anaemia (AIHA), by Adult Haematology consultants only. This is an unlicensed use of a licenced product. TBA Background Responses to rituximab have occurred in small, open-label single-arm clinical trials in adult patients with various types of AIHA which were either previously untreated or refractory. When used in the second-line setting, the response rate in warm AIHA is 64% to 93%. Response rates in cold agglutinin disease (CAD) are slightly lower, ranging from 45% to 60%. The maximum duration of responses from the studies of warm and cold AIHA are 20 months and 11 months respectively. The median time to response for both warm and cold AIHA is 6 weeks. Effective from Summary of evidence Autoimmune Haemolytic Anaemia (AIHA) Rituximab costs approximately £6000 per course per patient. Date Policy to be reviewed by Contact for the policy Given the limited nature of the evidence for clinical and cost-effectiveness, it is essential that rituximab for AIHA is monitored in terms of usage and clinical outcomes to inform future review and decision making. September 2013 2 years Jo Alldred, Leeds Medicines Effectiveness Lead jo.alldred@nhs.net Notes • • • • • • Disease AIHA is a rare condition with a small patient population base. It is divided into warm and cold disease Warm AIHA is responsible for 48-70% of cases. Annual incidence is in the region of 1-3 new cases per 100,000. Patients who are refractory to treatment have a reduced quality of life and increased mortality. Treatment The current management of AIHA is with steroids first line. Warm AIHA This responds to corticosteroids as first line therapy in 70-80% of patients. 15-20% of new cases will achieve complete remission but the remainder will require maintenance treatment. For those patients with an inadequate response to steroids, second-line treatment is usually splenectomy, which is associated with an initial response rate of approximately 50%. Treatment options for patients who fail to respond to, relapse after or are unable to undergo a splenectomy are limited. Cytotoxic and immunosuppressant drugs, such as cyclophosphamide, azathioprine and ciclosporin give a 40–60% response rate. However, these treatments may be associated with serious side effects. The effectiveness of other options, such as IVIG, plasmapheresis and danazol, is controversial. • Cold AIHA (CAD) is refractory to conventional AIHA treatments First line therapy is with steroids +/or blood transfusions For those with an inadequate response, immunosuppressants are used second line. Other options include plasmapheresis although this is often inadequate • Rituximab is a chimeric monoclonal antibody directed against the CD20 antigen. Studies have found it to be effective as a second line treatment for managing AIHA Rituximab will be included as a treatment option for second line management of both cold and warm AIHA. The dose will be 375mg/m2 once weekly for 4 weeks. • • • • • • • • • • • • Clinical Evidence There are no NICE or SMC guidelines for the use of Rituximab in AIHA, and most evidence are based on retrospective studies (Level V evidence) due to the small patient numbers. A review in 2008 concluded that rituximab was an effective alternative to splenectomy + / or chemotherapy in warm AIHA (1). Further review of retrospective studies demonstrated a response rate of 82% to rituximab. It suggested retreatment may be required every 1-3 years (2) A study in 2007 treated 11 patients with Rituximab. 8 achieved complete response (CR) and 3 achieved partial response (PR). All patients were maintaining CR / PR at a median follow up of 604 days (3) In a single-arm, open-label, prospective, multicentre clinical trial administration of rituximab was effective for the treatment of chronic cold agglutinin, autoimmune haemolytic anaemia (CAD). The overall response rate was 45% (n=20) with one CR and 8 with PR. Patients with idiopathic CAD accounted for one CR and 3 PR (4 of 13; 31%), while patients with secondary CAD accounted for 5 PR (5 of 7; 71%). Median time to maximal response was 3 months (range, 1-5 months). Median duration of response was 6.5 months (range, 2-10 months). Six patients relapsed within 48 weeks of follow-up. (4) In 2006, a population based retrospective study of 86 patients receiving a variety of treatments for CAD showed rituximab as the only treatment to to induce a complete response in any patient. The overall response rate for rituximab patients was 60%, with 10% achieving CR.(5) Monitoring and response Rituximab has a good side effect profile, a reduced impact on the patient’s quality of life and durable response is seen. Rituximab demonstrates a good safety profile. Most patients tolerated treatment well. Adverse reactions are limited to infusionrelated reactions. Neutropenia complicated by sepsis occurred in some patients, especially in immune-compromised and heavily pre-treated patients. Budget impact LTHT predict 3-5 Leeds patients per year will be treated with Rituximab for AIHA The cost of one cycle of Rituximab is approximately £6000. Therefore the cost to Leeds will be £18000 - £36,000 per year. The cost of a splenectomy is approximately £4000 (to check). Post-splenectomy, patients require lifelong antibiotic prophylaxis and vaccination programme The earlier use of rituximab in the longer term may result in reduced day unit attendance and reduced nursing time. References (1) Garvey B, Rituximab in the treatment of autoimmune haematological disorders, BJH, 2008,141, p149-169 (2) Dierickx D et al, Rituximab in auto-immune haemolytic anaemia and immune thrombocytopenic purpura: a Belgian retrospective multicentric studyJ Intern Med. 2009 Nov;266(5):484-91 (3) D'Arena G et al, Rituximab for warm-type idiopathic autoimmune hemolytic anemia: a retrospective study of 11 adult patients. Eur J Haematol. 2007;79(1):53-58. (4) Schollkopf C, Kjeldsen L, Bjerrum OW, et al: Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma 2006; 47: 253-260. (5) Berentsen et al, Primary chronic cold agglutinin disease: A population based clinical study of 86 patients. Haematologica. 2006;91(4):460–466.