The BCR study
advertisement

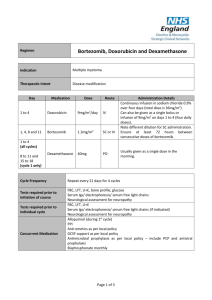
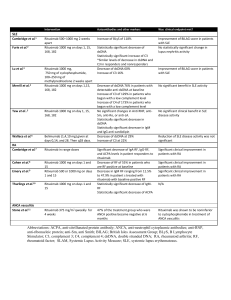
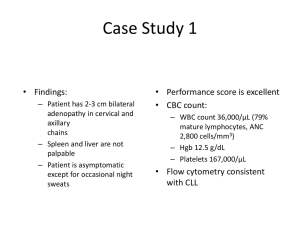
Waldenstrom’s: The Future Barts Cancer Institute Rebecca Auer r.l.auer@qmul.ac.uk www.cancer.qmul.ac.uk WM treatment • • • • • WM1 recently closed No other UK trials No standard treatment Difficult to achieve CR New agents Development pathway Novel strategies • Combinations including rituximab and/or bortezomib • Novel anti-CD20 Abs / proteasome inhibitors • Bendamustine • Novel signal inhibitors Everolimus Perifosine • Epigenetic modifiers Panobinostat • Immunomodulators IMiDs • Stem cell transplantation The BCR study Waldenstrom’s macroglobulinemia is somewhat similar to two other types of cancer, multiple myeloma (plasma cell cancer) and non-Hodgkin's lymphoma (a group of cancers of lymphocytes). Bortezomib Rituximab plasma cells B cells Bortezomib in WM • Predominantly in phase II trials in the relapsed or refractory setting • Alone or in combination • Rapid responses • As a salvage treatment option - Fourth International Workshop on WM treatment recommendations Rituximab • Minimal myelosuppression • Single agent RR 40-50% • Combination – chemotherapy – IMiDs Bortezomib & Rituximab in WM • Barts study in relapsed lymphoma – 9 of 10 patients with WM responded • 2 studies in USA in untreated WM – BDR twice a week – BR once a week 83% responded 65% responded Complete response/near-complete response = 22% A phase II trial of bortezomib, rituximab and cyclophosphamide in patients with symptomatic, untreated Waldenstrom macroglobulinemia • To determine the efficacy and safety of bortezomib, rituximab and cyclophosphamide • Symptomatic untreated WM • IV Bortezomib 1.6 mg/m2 on days 1, 8, 15 • Oral Cyclophosphamide 250 mg/m2 on days 1, 8, 15 • IV Rituximab 375 mg/m2 d1, 8, 15, 22 of cycles 2 and 4 – this will be repeated every 28 days for 6 cycles in responding patients. • 1° endpoint: Response rate • 2° endpoint: Toxicity, complete response rate, duration of response, speed of response, time to next treatment, progression free survival Study design • • • • Run in phase 6 patients Multicentre phase 33 patients Recruit over 2 years 6 centres – Barts, Leeds, Mid-Yorkshire, Heartlands, King’s, UCH, Plymouth • Plan to follow on with a phase III – BCR versus FCR Randomised phase II FCR BCR v or DCR Possibility of s/c bortezomib Side effects • Bortezomib neurological • Rituximab allergic / infections • Cyclophosphamide low blood counts Assessments • Blood tests every cycle • Bone marrow and CT scans at start, midway, at completion • Blood and BM assays to look for better markers of response • Research samples to look at some of the genetic & protein changes in WM Timelines • • • • • Application to CRUK Decision by CRUK Expectation open Duration recruitment Duration follow up Aug 2010 Nov 2010 May 2011 2 years 5 years April 2011 July 2011 Jan 2012 2 years 5 years New proteasome inhibitors • s/c Bortezomib – less neurotoxicity but as active • Carfilzomib – phase I data – no grade 3/4 neuropathy – activity • Marizomib – phase I studies recruiting Main mechanisms of action of rituximab and ways to increase its clinical efficacy Cartron, G. et al. Blood 2004;104:2635-2642 Copyright ©2004 American Society of Hematology. Copyright restrictions may apply. Novel anti-CD20 Abs • GA101 • Ofatumumab And other Abs to other proteins eg. Belimumab Bendamustine StiL Group - Rummel • BR versus R-CHOP first line n=549 – WM n=42 – ORR similar BUT CR, PFS, TTNT all significantly better with BR – Progressive disease in 2/23 BR versus 7/17 R-CHOP – Less grade 3/4 neutropenia with BR StiL Group - Rummel • BR versus FR relapse – BR higher ORR 83.5% v 52.5% CR 38.5% v 16.2% – grade 3/4 neutropenia similar n=219 PI3K/Akt/mTOR cell signalling pathway Overactive in WM cells Perifosine Everolimus Everolimus • Oral • ORR – 70% – PR 42% MR 28% • Median PFS and duration response not reached • Toxicities – Grade 3 or higher in 56% – Lung toxicity in 10% Perifosine • Oral • ORR - 35% • Median PFS 12.6 months • Toxicities – cytopenias – GI – Arthritis flare IMiDs IMiDs • Thalidomide + rituximab – dose reductions required in all patients – neuropathy • Lenalidomide + rituximab – study discontinued due to unexpected clinically significant anaemia • Pomolidomide HDACI Eg. Panobinostat Open studies Ofatumumab anti-CD20 monoclonal Ab Panobinostat epigenetic - HDACI Everolimus + BR mTOR inhibitor Belimumab monoclonal Ab Pomolidomide ImiD Waldenstrom’s: The Future Chemotherapy Monoclonal Ab Biologic agent