- Dr. Robert Fox
advertisement

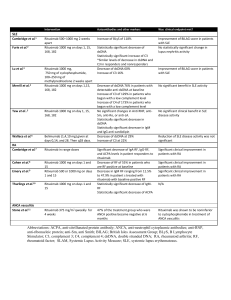
SJOGREN’S SYNDROME: Theory to Practice Robert I. Fox, M.D., Ph.D. Scripps Memorial Hospital Scripps/XiM Medical Center La Jolla, California USA robertfoxmd@mac.com Goals-1: 1) There are no FDA approved drugs for the systemic manifestations of Sjogren’s syndrome 2) Therefore, expert opinion must be used to choose therapies based on literature 3) These recommendations are summarized in my new chapter in UpToDate Goals-2 3. The existing treatment borrows from RA and SLE in the use of DMARDs, as well as interstitial pneumonitis and lymphoma 4. The most widely used biologic is rituximab, although not approved by FDA. 5. The question is when to use DMARD or Biologic Agent Goals-3 The most challenging issues for therapy 6. Neurologic Manifestations—including peripheral neuropathy, ganglionopathy, and central nervous involvement 7. Fatigue and cognitive loss 8. Lympho-proliferative swelling and possible lymphoma All slides are available and can be downloaded from my website using your desktop computer robertfoxmd.com (although these may not be accessed by iPhone due to our web security) 5 “Old” Consensus Criteria, 2002 called the American-European Consensus Group Criteria (AECG) Evidence of a systemic autoimmune cause for the dryness-– Positive anti-Ro (SS-A or SS-B antibody) – Positive minor salivary gland biopsy (focus score >1) A new consensus criteria is being developed with slightly different features It will be called the SICCA-AECG criteria ESSDAI- European SS Activity Index (to assess if therapy working) • Weighted domains to give a total score— the Sjogren’s equivalent to ACR-50 for RA. • The validated ESSDAI activity score has been the accepted outcome measure of FDA clinical trials. • Clinical significance is 3.5 units of change Systemic Manifestations • • • • • • Steroids work but have side effects. DMARDs to taper or replace steroids. Hydroxychloroquine Methotrexate, Azathioprine Mycophenolic acid mofetil We are interested in Sirolimus (rapamycin) Biologics Studied in SS • Anti-CD20 (rituximab)* –most widely used in SS although FDA approved • Belimumab (BAFF)-has been disappointing in SS • Abatacept (CD40 L)-Phase II safety good— improved ESSDAI but no control arm • TNF antagonists shown not useful Rituximab • Most widely used biologic in SS (ACR 2013 abstracts). • Used in response to extraglandular manifestations such as persistent glandular swelling, pneumonitis, mixed cryoglobulinemia. • Not approved by FDA. EYE DRYNESS results in the clinical appearance of keratoconjunctivitis sicca (KCS) characteristic of Sjogren’s Syndrome Artificial tears Punctal occlusion Ocular lubricants Treat blepharitis first Topical cyclosporin Topical “soft” steroids often Used if not able totolerate Restsis alone Systemic Ocular Often need DMARDs • Topical or intra-ocular steroids • Recurrent uveitis –may get by with azathioprine or cell cept • Retinal vasculitis-may need rituxan or cyclophosphamide • Watch out for ocular herpetic lesions Rash distinct from SLE (erythema annulare) the “old” subacute SLE which is negative for complement staining Therapy of skin lesions • Hydroxychloroquine for E. annulare or methotrexate if psoriaform • Vasculitis (usually small vessel) may need methotrexate • Vasculitis of mixed cryoglobulinemia responds to rituximab (indication approved by FDA) • Mono-neuritis multiplex (medium vessel) may also require cyclophosphamide Arthritis distinct from RA (Jaccoud’s like or erosive OA) Hydroxychloroquine, Methotrexate, Rituximab (less successful with azathioprine or leflunomide limited by leukopenia) Parotid swelling (after rule out infection and lymphoma, steroids and rituximab) Lymphocytic Interstitial Pneumonitis Bi-basilar on CXR Prominent Cystic on CAT Lymphocytes on biopsy Lung involvement (after rule out TBC, MAI, Lymphoma) • Interstitial pneumonitis—steroid and Mycophenolic acid; have avoided MTX due to MTX lung • Rituximab DeVic’s Syndrome: Transverse Myelitis Neuromyelitis Optica After rule out infection, treatments with cytoxan and or rituximab. Maintenance with azathioprine May need to treat like multiple sclerosis—new options approved Lymphocytic Interstitial Nephritis (steroids, mycophenolic acid, rituximab) SUMMARY-1 The American European Consensus criteria: •Subjective symptoms of dryness •Objective evidence of autoimmune process such as a positive antibody to SS-A or RF •Positive minor salivary gland biopsy SUMMARY-3 Additional Differential Diagnosis include: • Celiac disease • Hepatitis C and HIV • Sarcoidosis, IgG4-related disease • Tuberculosis, Syphilis, and Leprosy • Fibromyalgia with incidental autoantibodies SUMMARY-5 Recognize systemic (extraglandular) sites –Rule out infections and begin treatment with DMARDs to spare steroids. –DMARDs similar to use in SLE. –Hydroxychloroquine –Methotrexate, Azathioprine, mycophenolic acid SUMMARY-7 • Our treatment of fatigue in SS remains unsatisfactory, and represents a great therapeutic challenge for the next decade. • Later, we can discuss our approach to this problem in collaboration with Salk Institute and our research institute. Thank you for your time and attention We are still missing key targets in the pathogenesis of fatigue and the adrenal-hypothalmic axis. • In both SS and SLE, we can lower the cytokine with biologics, but the patient still feels little improvement. • This will be the focus of future direction for therapy. Information on website Benign manifestations include: • • • • Dry and painful eyes Dry and painful mouth Myalgias, arthralgia, fatigue Impaired cognition (executive function)— trying to distinguish “fibromyalgia” from “depression” Differential Diagnosis of SS-3 • The antibody to Ro (SS-A) or La (SS-B) do not fulfill criteria for SLE. • Many older patients labeled with mild SLE actually have SS. • Many patients in Hematology clinic with mixed cryoglobulinemia, hemolytic anemia or ITP actually have SS. Is Sjogren’s just SLE with 4/5 SLE Criteria? • Different antibody profile (antiSSA/B) are not criteria for SLE; • SS is more organ specific – (salivary/lacrimal gland) and more lymphoproliferative. Why is Sjogren’s not just SLE with 4/5 Criteria? 1. Interstitial pneumonitis (not pleurisy), interstitial nephritis (not glomerulonephritis) 2. Higher frequency of lymphoma 3. Genome Screens support this with Homing receptors found in SS but not SLE (CXCR5) Summary-1 1. Functional circuit needs to be considered when assessing “benign” symptoms of corneal or oral pain. 2. Symptoms of oral/ocular pain do not correlate with markers of systemic inflammation (ESR/CRP) because the events are contained within the brainstem and cortex. Moulton et*. Al used fMRI in SS patients with chronic ocular pain using fMRI of nociceptive pain have been studied Cortical regions that activate with ocular pain signal at “benign stimuli levels” occur only in chronic SS patients with severe pain *Moulton EA, Becerra L, Rosenthal P, Borsook D. An Approach to Localizing Corneal Pain Representation in Human Primary Somatosensory Cortex. PloS one 2012;7:e44643. Dry and Painful Mouth-1 • If you thought that Dentists did not care about SS, then wait until you see their Dental Care Plans -The answer to all problems is a $25,000 tooth implant. Dry and Painful Mouth-2 • Must treat underlying oral candida (which is erythematous spots on roof of mouth) before anything will work. • Candida often lurks under dentures– • Patients would rather run naked through clinic than remove a denture. Dry and Painful Mouth-3 • Angular cheilitis the most obvious hint. • Treatment of oral candida is a slow process involving multiple steps. • Use website for education. We are also looking at additional targets of interest • Chemokines and their receptors (CCR) on vascular cells and lymphocytes • TLR receptors: SLAC-15 that links Toll receptor and type 1 IFN • Methylation modulators and siRNA • Neural mediator circuits: • Receptors on cornea--substance P (TRPV1), VIP and CGRP pain receptors • TRPM8, TRPA1, and CGRP in trigeminal ganglion neurons • Trigeminal ganglion neurons- MCP-1, MIP-2, • CCR and CCL at the blood brain barrier CCR and Blood Brain Barrier The tsp-null mouse allows us to look at the interaction of peripheral inflammation and microglial cells • Activation of microglial cells through mTor/AKT • In absence of thrombospondin, constitutive activation of Th17 and IFN-g activates microglial cells • Nociceptive (pain) pathway occurs through smad3 and non-smad pathways that involve mTor/AKT pathways in cranial nerve V Thank you for inviting us. Robert I. Fox, M.D., Ph.D. http://www.robertfoxmd.com RobertFoxMD@mac.com