Renal and Hepatic Toxicology
advertisement

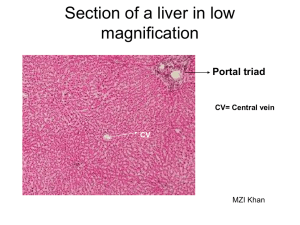
Introduction to Toxicology EV 460/660 & BI 460/660 Fall 2014 Toxic Effects on the Kidney Brief Review of Kidney Structure and Function 1. 2. 3. 4. . Functions of kidneys --excretion of water soluble wastes and xenobiotics -- regulation of osmotic pressure of body fluids – water balance and concentration of inorganic salts -- regulation of blood volume and pressure -- regulation of pH of body fluids -- endocrine functions – erythropoietin, vitamin D3, renin—angiotensin -- gluconeogenesis Renal blood flow Renal macrostructure – capsule, cortex, medulla (pyramids), pelvis Processes of urine formation – filtration, tubular reabsorption, tubular secretion Nephron structure and function and associated blood vessels -- renal corpuscle (glomerulus and capsule) – filtration via trilaminar molecular sieve -- proximal tubule – obligatory reabsorption of filtrate, some secretion -- loop of nephron – countercurrent multiplier established corticomedullary osmotic concentration gradient, allows production of hypertonic urine -- distal tubule – further reabsorption and secretion -- collecting duct – final water reabsorption Toxic Effects on the Kidney 1. 2. 3. 4. 5. 6. Particular susceptibility of kidneys to toxic injury -- tremendous renal blood flow -- solute concentration mechanism increases concentration of chemicals in tubular fluid -- high transport of chemicals into tubular cells -- biotransformation of parent compounds to toxic metabolites -- affinity of some toxicants/toxins for kidney Large variety of mechanisms of toxic action -- formation of reactive oxygen species -- inactivation of sulfhydryl containing enzymes -- impaired mitochondrial function and energy metabolism -- toxicant-induced autoimmune attack -- alterations in fluid flow (permeability alterations, physical blockage) -- alterations in membrane polarity -- rupture of intracellular lysosomes -- alterations in calcium ion homeostasis -- alterations in sodium, potassium, and chloride ion homeostasis and cell volume Most common site of toxic injury is the proximal tubule – reasons for susceptibility include -- tremendous movement of materials through tubular epithelium due to reabsorption -- selective accumulation of xenobiotics -- cytochrome P-450 activity largely restricted to proximal tubule (bioactivation) -- high metalothionein content -- high susceptibility to ischemia and oxidative stress Glomerulus also site of toxic action – but relatively few compared to proximal tubule, actions include -- alterations in permeability -- alterations in membrane charge -- autoimmune attack and physical blockage by circulating immune complexes Remainder of nephron structures relatively uncommon sites of toxic action Some examples of nephrotoxic toxicants A. Heavy metals – damage proximal tubule – major metals Hg, Cd, and Pb, several others B. Halogenated hydrocarbons – damage proximal tubule – exs. chloroform, carbon tetrachloride, bromobenzene C. Some antibiotics, herbicides, organic solvents, and mycotoxins Introduction to Toxicology EV 460/660 & BI 460/660 Fall 2014 Toxic Effects on the Liver Brief Review of Liver Structure and Function 1. 2. 3. 4. Functions of liver -- biotransformation of toxins/toxicants (review previous class notes), production of bile and biliary excretion, enterohepatic circulation of bile, nutrient storage and transformation, protein synthesis (including transport proteins and clotting factors), urea formation Hepatic blood supply – hepatic artery and hepatic portal vein Hepatic structure – lobes, lobules and acini -- lobule organization – central vein, sinusoids, canaliculi, hepatocytes (parenchymal cells) -- periportal , midzonal and centrolubular regions -- acinus organization – zones 1, 2 & 3, functional gradient between zones -- zone 1 – blood higher in O2 and bile, cells higher in mitochondria and glutathione -- zone 3 – blood lower in O2 and bile, cells higher in P450 enzymes Regenerative capacity of liver Toxic Effects on the Liver 1. 2. Particular susceptibility of the liver to toxic insult -- high blood flow, perfusion from both hepatic artery and hepatic portal vein -- “first pass” effect, e.g., many toxins/toxicants enter body through ingestion and many of these pass directly to liver via portal vein -- high biotransformation activity of liver – increased exposure to toxic metabolites of bioactivation -- “multiple pass” effect via enterohepatic recirculation of toxins/toxicants -- high protein content of liver, high nutrient storage of liver, high energy demands of hepatocytes Types of toxic effects on the liver A. Fatty liver (steatosis) -- liver is site of synthesis, storage, and release of lipids -- fatty liver is increase in hepatic lipid content to greater than 5% by weight-- common response to acute exposure to hepatotoxins, often reversible, does not necessarily lead to impaired hepatic function and hepatic cellular necrosis -- multiple mechanisms of action – including increased triglyceride synthesis, decreased triglyceride secretion, and decreased synthesis or secretion of very low density lipoproteins -- example toxicants inducing fatty liver- carbon tetrachloride, ethanol B. Hepatic necrosis -- death of hepatocytes -- multiple degenerative cellular changes including cytoplasm, ER, mitochondria, nucleus, and plasma membrane -- cellular death is described as: -- focal (randomly distributed, isolated cluster of cells) -- zonal (either zone 1—periportal or zone 3 – centrolubular), zone 3 damage is most common -- diffuse/massive – (panlobular or panacinar) -- lipid peroxidation forming free radicals is a major mechanism, particularly for toxicants that are bioactivated by P450 reactions, particularly for toxicants causing zone 3 damage -- free radicals damage cell and organelle membranes, impair mitchondrial and ER function, and allow excessive calcium influx -- example toxicants inducing hepatic necrosis -- carbon tetrachloride, bromobenzene, chloroform and other halogenated hydrocarbons, ethanol, acetaminophen C. Cirrhosis -- results from chronic liver injury by hepatotoxicants, non-reversible -- repeated death and regeneration of hepatocytes accompanied by fibroblast production of excessive collagen fibers, architecture of liver is disrupted by fibrous scar tissue and irregular cords of regenerated hepatocytes -- decreased liver function due to progressive decrease in hepatocytes and impaired blood flow -- role of inadequate nutrition in development of cirrhosis is controversial -- example toxicants inducing cirrhosis – ethanol, arsenic D. Cholestasis -- impaired bile production or bile flow, results in jaundice, typically drug-induced