AP Chemistry Practice Exam
advertisement

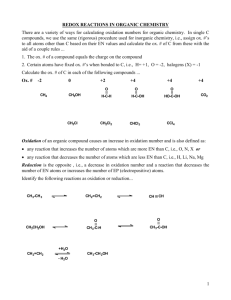
AP Chemistry Chapter 20 Exam Multiple Guess: Name ___________________________ Questions 1-5: The set of lettered choices is a list of ions that participate in oxidation reduction reactions. Select the one lettered choice that best fits each numbered description. A choice may be used once, more than once or not at all. A) C2O42B) CrO42C) MnO4D) NO3E) Fe2+ 1. undergoes oxidation such that its oxidation number changes from +3 to +4 ____________________ 2. includes the species with a +7 oxidation number _____________________ 3. reacts with a reducing agent in acid solution such that the oxidation number of the metal becomes +3 _______________________ 4. when oxidized, forms a cation with a +3 oxidation number _____________________ 5. reacts with a reducing agent in acid solution to form a brown gas ________________ 6. Mg + 2Ag+ Mg2+ + Ag Eo = 3.17 V For the reaction above in one experiment, the observed value for E is +3.00 V. Which statement(s) apply to this cell? I. Addition of more oxidizing agent will increase the value of E. II. Addition of products will decrease the value of Eo. III. As this reaction proceeds, the value of E will decrease and the value of Eo will remain the same. A) I only B) II only C) I and II only D) I and III only E) I, II, and III Questions 7 and 8: Balance the following equation using redox techniques. …H+ + …Br- + …MnO2 …Mn2+ + …Br2 + …H2O 7. What is the sum of the coefficients in this balanced equation? A) 12 B) 11 C) 10 D) 8 E) 6 8. What is the reducing agent in this reaction? A) H+ B) BrC) MnO2 D) Mn2+ E) Br2 Questions 9-11: A 1.0 M solution of KI is undergoing electrolysis. A few drops of phenolphthalein solution are added. The current is supplied at the rate of 2.5 amperes. 9. Which describes the appearance of the solution after the reaction has proceeded for a few minutes? At the anode At the cathode A) colorless pink B) brown pink C) brown colorless D) pink colorless E) colorless colorless 10. Which describes the behavior of the K+ ions in this system? A) K+ ions migrate, as spectator ions, toward the cathode. B) K+ ions migrate toward the anode where they become oxidized to K(s). C) K+ ions migrate toward the anode where they become reduced to K(s). D) K+ ions migrate toward the cathode where they become oxidized to K(s). E) K+ ions migrate toward the cathode where they become reduced to K(s). 11. Which best describes the behavior in the chamber containing the electrode at which bubbles of gas are observed? A) O2 is produced as the pH of the solution increases. B) O2 is produced as the pH of the solution decreases. C) H2 is produced as the pH of the solution increases. D) H2 is produced as the pH of the solution decreases. E) O2 is produced as the pH of the solution remains the same. 12. In the reaction: 2KMnO4 + 3H2SO4 + 5H2S 5S + 2MnSO4 K2SO4 + 8H2O the oxidation number of Mn changes from A) 0 to -2 B) +7 to -8 C) -2 to 0 D) -2 to +2 E) +7 to +2 13. The order of chemical activity of three metals is L>M>N. Which describes the behavior of these metals? I. Atoms of L can reduce atoms of N. II. Atoms of L can reduce cations of N. III. Atoms of M can reduce cations of L. A) I only B) II only C) I and II only D) II and III only E) I, II, and III only 14. When the value of Eo for a standard galvanic cell is greater than zero, which ranges apply to Go and Keq for the cell reaction? Go Keq A) <0 >1 B) <0 <0 C) >0 >1 D) <0 >0 but <1 E) >0 >0 but <1 15. Which describes the oxidation number of nitrogen in each ion of NH4NO2? A) Both nitrogen atoms have the same oxidation number with the same sign. B) Both nitrogen atoms have the same oxidation number with the opposite sign. C) The oxidation numbers of the two nitrogen atoms are +4 and -5, respectively. D) The oxidation numbers of the two nitrogen atoms are -3 and +4, respectively. E) The oxidation numbers of the two nitrogen atoms are -4 and +5, respectively. Free Response 1. The compound NaI dissolves in pure water according to the equation NaI (s) Na+ (aq) + I- (aq). An electric current is applied to a 1.0 M NaI solution. a) Write the balanced oxidation half-reaction for the reaction that takes place. b) Write the balanced reduction half-reaction for the reaction that takes place. c) Which reaction takes place at the anode, the oxidation reaction or the reduction reaction? d) All electrolysis reactions have the same sign for Go. Is the sign positive or negative? Justify your answer. 2. Answer the following questions about the setup and operation of a galvanic cell. a) One item is missing from this electrochemical cell that prevents it from functioning. Identify that item by drawing it onto the diagram. Label the missing item. b) Once this cell is operating, a redox reaction occurs. i) Which electrode, Cu or Zn, is the cathode? Explain. ii) Write the reaction for the half-cell containing zinc. iii) Calculate the voltage Eo for this cell. c) Set up the equation to calculate E in volts for the cell when the molarity of Cu2+ in the left half-cell is reduced to 0.10 M. Assume standard temperature. Label each factor in the equation with proper units. d) Suppose that the temperature of the original galvanic cell is decreased (from standard temperature) to 273 K. Would the voltage decrease, stay the same, or increase? Explain. e) What would be the effect on the voltage if the metal electrodes were doubled in mass? Explain. f) What would be the effect on the voltage if a solution of NaOH were poured into the Zn/Zn2+ halfcell? Explain. g) What would be the effect on the voltage if a solution of 10.0 M Zn(NO3)2 were poured into the Zn/Zn2+ half-cell? Explain. 3. Water was electrolyzed, as shown in the diagram above, for 5.61 minutes using a current of 0.531 ampere. A small amount of nonreactive electrolyte was added to the container before the electrolysis began. The temperature was 298 K and the atmospheric pressure was 1.00 atm. a) Write the balanced equation for the reaction that took place at the anode. b) Calculate the amount of electric change, in coulombs, that passed through the solution. c) Why is the volume of O2 (g) collected different from the volume of H2 (g) collected, as shown in the diagram? d) Calculate the number of moles of H2 (g) produced during the electrolysis. e) Calculate the volume, in liters, at 298 K and 1.00 atm of dry H2 (g) produced during the electrolysis. f) After the hydrolysis reaction was over, the vertical position of the tube containing the collected H2 (g) was adjusted until the water levels inside and outside the tube were the same, as shown in the diagram below. The volume of gas in the tube was measured under these conditions of 298 K and 1.00 atm, and its volume was greater than the volume calculated in part (e). Explain. .