The Molar Volume
advertisement

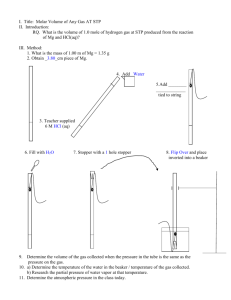
The Molar Volume Chapter 10 – Blue Book HW: #1-17, 19-20 & 23-24 #1-11 DUE NEXT CLASS!!! I. Avogadro’s Principle & Molar Volume A. Relationship between the mass of a gas & its volume: 1. Equal volumes of all gases, measured under the same conditions of P & T, contain the same number of particles. a. 1 mole of any gas @ STP contains 6.02 x 1023 particles b. 1 mole of any gas has a mass = to its molecular mass *EXAMPLES:* ** 1 mole N2 = 28.0 g N2 = 6.02 x 1023 molecules of N2 ** 1 mole of CO2 = 44.0 g CO2= 6.02 x 1023 molecules of CO2 c. 1 mole of any gas (@STP) = molecular mass = 22.4 dm3 of the gas Examples 1. A sample of gas has a mass of 1.248 g and occupies 300.0 cm3 at STP. What is the molecular mass of this gas? Solving Process: The molecular mass of the gas is equal to one mole of the gas. Start with the relationship between volume & mass & calculate the mass of one mole of the gas. All units must be divided out except g/mol. Examples 1. How many grams of CO2 will occupy a volume of 500 cm3 at STP? Remember: 1 mol CO2 = 22.4 dm3 CO2 at STP 1 mol CO2 = 44.0 g CO2 II. Molar Volume & Gases Collected Over Water A. We can apply what we know about molar volume to lab situations involving gases collected over water Example: A reaction produces 200 cm3 of oxygen over water and measured at 22 oC and 99.2 kPa. How many grams of the gas are produced? Assume water vapor pressure of 2.6 kPa at 22 oC. III. Ideal Gas Equation A. Combo of the four physical variables: pressure, volume, temperature & number of particles. 1. Equation: PV = nRT P = pressure in kPa V = volume dm3 T = temperature in K n = number of moles of a gas R = 8.31 (dm3 x kPa) / (mol x K)* (other values for R depending on the units) 2. n = m/M m = mass M = molecular mass Other ways to manipulate the Ideal Gas equation: 3. PV = (m/M)RT Example 3. A flask has a volume of 258 cm3. A gas with a mass of 1.475 g is introduced into the flask at a temperature of 300K and a pressure of 98.6 kPa. Calculate the molecular mass of the gas using the ideal gas equation. IV Gas Concentration A. Can be expressed many ways: 1. Percent by volume – volume of the given compound contained in 100 volumes of air (example air contains about 20% O2 by volume) 2. Parts per million (ppm) - # of mg of a compound per one kg of the whole. 3. Parts per billion (ppb) - # of μg (micrograms) per kg of the whole. Examples: 1 ppm is $.01 in $10,000 1ppb is $.01 in $10,000,000 1 ppm is 1mm in 1km 1 ppb is 1mm in 1000 km