Homework Assignment #1
advertisement

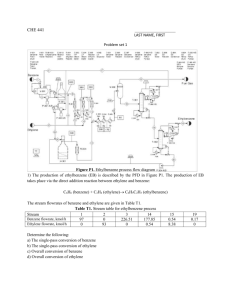
5310: Combustion Fundamentals Homework #1 Assigned: September 17, 2012 Due: October 4, 2012 Computer-based Problem: Write a computer code to solve for the adiabatic flame temperature and product composition for hydrogen-oxygen combustion over a range of equivalence ratios. The products of the reaction are H2O, H2, H, O2, O, and OH. Qualitatively and quantitatively compare your results with NASA CEA. Complete the following questions from An Introduction to Combustion, 3rd Edition: 2.5, 2.7, 2.8, 2.9, 2.12, 2.16, 2.18, 2.23, 2.27, 2.31, 2.37, 2.42, 2.45, 2.55 Complete the following questions using NASA CEA: 2.51, 2.52 Problems 1-9: Solve the problems completely. 1. Suppose that methane and air in stoichiometric proportions are brought into a calorimeter at 500 K. The product composition is brought to the ambient temperature (298 K) by the cooling water. The pressure in the calorimeter is assumed to remain at 1 atm, but the water formed has condensed. Calculate the heat of reaction. 2. Calculate the flame temperature of normal octane (liquid) burning in air at an equivalence ratio of 0.5. For this problem assume that there is no dissociation of the stable products formed. All reactants are at 298 K and the system operates at a pressure of 1 atm. Compare the results with those given in the graphs in lecture. 3. Carbon monoxide is oxidized to carbon dioxide in an excess of air (1 atm) in an afterburner so that the final temperature is 1,300 K. Under the assumption of no dissociation, determine the air-fuel ratio required. Report the results on both a molar and mass basis. For the purposes of this problem assume that air has the composition of 1 mol of oxygen to 4 mol of nitrogen. The carbon monoxide and air enter the system at 298 K. 4. The exhaust of a carbureted automobile engine, which is operated slightly fuel-rich, has an efflux of unburned hydrocarbons entering the exhaust manifold. Assume that all the hydrocarbons are equivalent to ethylene (C2H4) and all the remaining gases are equivalent in inert nitrogen (N2). On a molar basis there are 40 mol of nitrogen for every mole of ethylene. The hydro-carbons are to be burned over an oxidative catalyst and converted to carbon dioxide and water vapor only. In order to accomplish this objective, ambient (298 K) air must be injected into the manifold before the catalyst. If the catalyst is maintained at 1,000 K, how many moles of air per mole of ethylene must be added if the temperature of the manifold gases before air injection is 400 K and the composition of air is 1 mol of oxygen to 4 mol of nitrogen? 1 5. A combustion test was performed at 20 atm in a hydrogen-oxygen system. Analysis of the combustion products, which were considered to be in equilibrium, revealed the following: Compound H2O H2 O2 O H OH Moles 0.493 0.498 0 0 0.02 0.005 1.016 Mole Fraction 0.485236 0.490157 0 0 0.019685 0.004921 0.999999 What was the combustion temperature in the test? 6. Whenever carbon monoxide is present in a reacting system, it is possible for it to disproportionate into carbon dioxide according to the equilibrium: 2CO C s CO2 Assume that such an equilibrium can exist in some crevice in an automotive cylinder or manifold. Determine whether raising the temperature decreases or increases the amount of carbon present. Determine the Kp for this equilibrium system and the effect of raising the pressure on the amount of carbon formed. 7. Determine the equilibrium constant Kp at 1,000 K for the following reaction: 2CH 4 2H 2 C2 H 4 8. The atmosphere of Venus is said to contain 5% carbon dioxide and 95% nitrogen by volume. It is possible to simulate this atmosphere for Venus reentry studies by burning gaseous cyanogen (C2N2) and oxygen and diluting with nitrogen in the stagnation chamber of a continuously operating wind tunnel. If the stagnation pressure is 20 atm, what is the maximum stagnation temperature that could be reached while maintaining Venus atmosphere conditions? If the stagnation pressure was 1 atm, what would the maximum temperature be? Assume all gases enter the chamber at 298 K. Take the heat of formation of cyanogen as (Hf°)298 = 374 kJ/mol. 9. A mixture of 1 mol of N2 and 0.5 mol O2 is heated to 4,000 K at 0.5 atm, affording an equilibrium mixture of N2, O2, and NO only. If the O2 and N2 were initially at 298 K and the process is one of steady heating, how much heat is required to bring the final mixture to 4,000 K on the basis of one initial mole of N2? 2 Problems 10-14 require iteration and are more time consuming. Solve Problem 12 completely, and only set-up (write-down all the needed equations and the solution procedure) Problems 10, 11, 13, and 14. For Problem 14, note that UDMH (unsymmetrical dimethyl hydrazine) has chemical formula C2N2H8. 10. Calculate the adiabatic decomposition temperature of benzene under the constant pressure condition of 20 atm. Assume that benzene enters the decomposition chamber in the liquid state at 298 K and decomposes into the following products: carbon (graphite), hydrogen, and methane. 11. Calculate the flame temperature and product composition of liquid ethylene oxide decomposing at 20 atm by the irreversible reaction: C2 H 4Oliq aCO bCH 4 cH 2 dC2 H 4 The four products are as specified. The equilibrium known to exist is: 2CH 4 2H 2 C2 H 4 The heat of formation of liquid ethylene oxide is (Hf°)298 = -76.7 kJ/mol. It enters the decomposition chamber at 298 K. 12. Liquid hydrazine (N2H4) decomposes exothermically in a monopropellant rocket operating at 100 atm chamber pressure. The products formed in the chamber are N2, H2 and ammonia (NH3) according to the irreversible reaction: N 2 H 4 liq aN 2 bH 2 cNH 3 Determine the adiabatic decomposition temperature and the product composition a, b, and c. Take the standard heat of formation of liquid hydrazine as 50.07 kJ/mol. The hydrazine enters the system at 298 K. 13. Gaseous hydrogen and oxygen are burned at 1 atm under the rich condition designated by the following combustion reaction: O2 5H 2 aH 2O bH 2 cH The gases enter at 298 K. Calculate the adiabatic flame temperature and the product composition a, b, and c. 14. The liquid propellant rocket combination nitrogen tetroxide (N2O4) and UDMH (unsymmetrical dimethyl hydrazine) has optimum performance at an oxider-to-fuel weight ratio of 2 at a chamber pressure of 67 atm. Assume that the products of combustion of this mixture as N2, CO2, H2O, CO, H2, O, H, OH, and NO. Write down the equations necessary to calculate the adiabatic combustion temperature and the actual 3 product composition under these conditions. These equations should contain all the numerical data in the description of the problem. The heats of formation of the reactants are: N2O4 (liquid) UDMH (liquid) or C2N2H8 (Hf°)298 = -2.1 kJ/mol (Hf°)298 = 53.2 kJ/mol The propellants enter the combustion chamber at 298 K. 4