HW1
advertisement

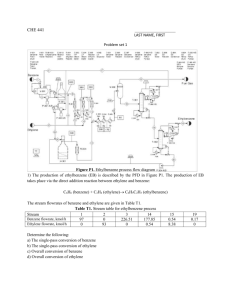
CHE 441 Answers to problem set #1 FIC 6 R-302 3 8 11 H-301 P-301 A/B R-303 R-301 7 hps 5 9 hps E-301 E-302 10 bfw bfw 12 14 13 Figure P1. Ethylbenzene process flow diagram 1) The production of ethylbenzene (EB) is described by the PFD in Figure P1. The production of EB takes place via the direct addition reaction between ethylene and benzene: C6H6 (benzene) + C2H4 (ethylene) C6H5C2H5 (ethylbenzene) The stream flowrates of benzene and ethylene are given in Table T1. Table T1. Stream table for ethylbenzene process Stream 1 2 3 14 Benzene flowrate, kmol/h 97 0 226.51 177.85 Ethylene flowrate, kmol/h 0 93 0 0.54 15 8.38 0.54 19 0.17 0 Determine the following: a) The single-pass conversion of benzene: 21.5% b) The single-pass conversion of ethylene: 99.4% c) Overall conversion of benzene: 91.2% d) Overall conversion of ethylene: 99.4% 2) a) In Contra Costa County, California, the median indoor airborne concentration of chloroform (CHCl3) was found to be 0.4 μgm-3. Convert this concentration to a mole fraction in parts per billion (ppb) assuming that T = 293oK and P = 1 atm. b) The same study estimated that the mean concentration of chloroform in drinking water is 42 μg/L. Convert this to a mass fraction in parts per billion (ppb). c) A typical adult inhales about 20 m3 of air and ingest about 2 L of water per day. Assuming that the concentrations given in (a) and (b) are appropriate, compare the exposure to chloroform via inhalation (μg inhaled per day) with the exposure via ingestion (μg swallowed per day). d) For tetrachloroethylene (C2Cl4), the study found a mean concentration in water of 0.10 μg/L and a mean concentration in indoor air of 2.1 μgm-3. Repeat part (c) for this species. (a) (b) (c) (d) 0.0803 ppb 42 ppb inhaled per day: 8 μg, swallowed per day: 84 μg C2Cl4: inhaled per day: 42 μg, swallowed per day: 0.2 μg (3) A brochure from a municipal water supply agency lists the following average concentrations of selected ions in drinking water: Ions Fe3+ Ca2+ Mg2+ K+ Na+ Concentration (mg/L) 0.02 9.8 1.8 0.6 4.6 Compute the molarity and normality of each of these species. Note: # of moles of solutes mol Molarity = M = = volume of solution L # of equvalents of solutes eq Normality = N = = , volume of solution L where eq = |net charge|×moles and N = |net charge|×M Solution Ions Concentration (mg/L) Mw (g/mol) Net charge M (mol/L) N (eq/L) Fe3+ Ca2+ 0.02 56 3 3.57×10-7 1.07×10-6 9.8 40 2 2.45×10-4 4.90×10-4 Mg2+ 1.8 24 2 7.5×10-5 1.5×10-4 K+ 0.6 39 1 1.54×10-5 1.54×10-5 Na+ 4.6 23 1 2.0×10-4 2.0×10-4 4) The Bay Area Air Quality Management District comprises nine counties. The total land area is 6094 square miles. Assume that a steady uniform wind blows from west to east at 3 m/s through the air basin. What is the characteristic residence time of air in the air basin? Answers Residence time = (Volume/Flow rate) = 4.187×104 s = 11.6 h 5) The mass fraction of sulfate (SO42-) in seawater is 2.7 g/kg. The total mass of the oceans is 1.4×1021 kg. a) Compute the total mass of S in the oceans. b) Combine the results of (a) with the data below on sulfur flows to estimate the residence time of S in the oceans. Transport of S from atmosphere to ocean surfaces 160×1012 g/y River flow of S to the oceans 100×1012 g/y Answers a) Total mass of S in the oceans. 1.26×1021 g S b) Residence time of S in the oceans 4.85×106 years 6) Anaerobic digesters are commonly used for processing sludge in wastewater treatment facilities. They are generally operated at 35oC to optimize the rate of microbial conversion of sludge to methane and carbon dioxide. At a specific facility, the gases produced from this reaction are collected in a 1000 m3 gas relief chamber, which requires the gas to reach a pressure of 3 atm before release. a) Given that the temperature within the gas collection chamber is 35oC and that the composition of the gas is 65 percent methane (CH4) and 35 percent carbon dioxide (CO2), what mass (in kg) of each compound would be held within the container just as the release pressure (3 atm) is achieved? b) If the gas is allowed to cool to 25oC before entering the gas relief chamber, compute the new mass of each compound within the chamber prior to release. c) If the rate of gas production is 400 kg/d, what is the characteristic residence time of a gas molecule within a 25oC gas relief chamber operating with steady-state gas release? Answers Ideal gas constant = R = 82.05×10-6 m3 atm/molK a) Mass CH4 = 1.23×103 kg Mass CO2 = 1.83×103 kg b) Mass CH4 = 1.28×103 kg Mass CO2 = 1.89×103 kg c) Residence time = 7.91 days 7) Consider a box with a volume of 1 m3. The box is initially filled with dry air at a pressure of 1 atm and a temperature of 293oK. a) How many moles of air are in the box? b) Starting from the condition given in the problem statement, 0.5 mol dry air is replaced with 0.5 mol water. At equilibrium, assuming T = 293 K, what is the relative humidity in the box? c) Starting from the condition given in the initial problem statement, 2 mol dry air are removed and replaced with 2 mol water. At equilibrium, assuming T = 293 K, what is the relative humidity in the box? d) What is the total air pressure in the box for condition (c)? Answers -6 3 Ideal gas constant = R = 82.05×10 m atm/molK a) n = 41.596 moles b) RH = 52.57% c) RH = 100 % d) 0.975 atm 8) The following experiment is conducted to measure the Henry’s law constant of chloroform (CHCl3). A vessel with a volume of 2 L is half filled with water and then sealed, so it contains 1 L of water and 1 L of air at 1 atm and 293 K. One gram of liquid chloroform is injected into the water through a rubber septum. The contents are thoroughly mixed and then allowed to equilibrate. A sample of gas is extracted from the air space above the water. Using a gas chromatograph, it is determined that the mass concentration of chloroform in air is 89 mg/L. Determine the value of the Henry’s law constant in units of M/atm. Answers Henry's law constant = Cw,chloroform/Pchloroform = 7.63×10-3/0.0179 Matm-1 = 0.4263 Matm-1