017 Measuring Planck`s Constant Lab
advertisement

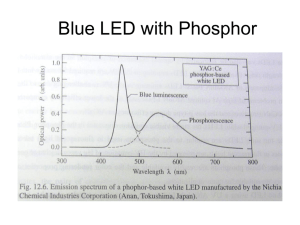
Determining Planck’s Constant (Adapted from Perimeter Institute’s and Arbor Scientifics worksheets by Nancy J. Easterly, edited by Stephen R. Skinner) Purpose: To demonstrate the quantization of light and to determine Planck’s constant, (h). Background Information: The energy lost by a single electron that passes through a lightemitting diode (LED) is converted into the energy of one photon. Materials: Jameco Electronics: Red LED – Part #206519 – Wavelength 660nm Green LED – Part #153154 – Wavelength 565nm Blue LED – Part #2006764 – Wavelength 468nm IR LED – Part #106526 – Wavelength 940nm 1 K Ohm Resistor – Part #690864 – Only needed if you use a 9V battery. Trim Potentiometer 1 K Ohm – Part #94693 2 “D” Cell batteries w/ holder Voltmeter Connecting leads Procedure: CAUTION: Do not stare directly at a brightly lit LED. Connecting the Circuit: Connect the circuit as pictured below. Note that the long end of the LED is the positive end and must be connected to the positive end of the battery or it will not turn on. The positive end of the battery is on the left hand side of the photo. Taking measurements: When the class is ready to take measurements the lights should be dimmed. Another way to obtain good results without dimming the lights is to shield the light from the LEDs with a film canister or piece of dark rubber tubing. 1. Using the potentiometer black dial face up, SLOWLY increase the potential difference across the blue LED by turning the potentiometer counterclockwise until the LED just begins to glow. You may need to try this a couple times before actually taking the measurement. It would be wise for each lab member to take the measurements so that an average can be obtained. 2. To take the measurement needed, connect the voltmeter (set on a scale of at least 3 V) between the legs of the LED. Record the potential difference in the data table. 3. Once you obtain the measurement, turn the potentiometer all the way clockwise, before proceeding to the next LED. Repeat steps 1 and 2 for the green, red and IR LEDs always turning the potentiometer all the way to clockwise before proceeding to the next LED. 4. To obtain the potential difference for the IR LED view the LED through a digital camera or cell phone to see when the IR LED lights up. The analysis: When we apply a large enough potential difference across a light-emitting diode, it emits photons that all have the same frequency. When the LED just begins to glow, the energy (E) lost by each electron as it passes through the LED is converted into the energy of a single photon. The energy lost by each electron is E = qV, where q is the elementary charge (1.6 x 10-19 C) and V is the potential difference across the LED. The energy (E) of a photon is E=hf where h is Planck’s constant and f is the frequency of the photon. Values for LEDs: IR = 940 nm, Red = 660 nm, Green = 565 nm, Blue = 468 nm Useful information: c =f q = 1.6 x 10-19 C c = 3.0 x 108 m/s h = 6.63 x 10-34 Js 5. Plot a graph of photon energy in Joules vs. frequency in Hertz. 6. Find the slope of the graph. What should the value of the slope be (include units)? 7. Calculate the relative error for your value of Planck’s Constant. Questions: 1. What does this experiment tell us about the energy of the different colored photons? Which LED requires the least amount of energy to light? The most? 2. If we had an LED that was for UV light, do you think it would take more or less energy to light up? Why? 3. Why is it that UV rays from the sun burn our skin more easily compared to the heat waves (IR) that come from a fire in a fireplace? Possible Data Table Color of LED Blue Green Red IR Wavelength Frequency Potential difference E = qV Determining Planck’s Constant Teacher Notes Students should not stare direct at LEDs when they are brightly lit. LEDs are safe when they just begin to glow, but the quickly become bright as the potential difference across them increases above the threshold value. Be especially careful with the blue LED as the upper part of its frequency spectrum runs in the UV region, which can cause permanent eye damage. Students should not stare at the blue LED even when it is dimly lit. LEDs can be destroyed if the current flowing through them is too large. If you choose to use a 9V battery instead of the 2-1.5V batteries, you will need to use a current limiting resistor. The purpose of the resistor connected in series with the LED is to limit the current flowing through the LED so the current is no more than 50 mA. The potentiometer can be destroyed if wired incorrectly. Students should be careful when wiring the potentiometer. If they wire it incorrectly, they can create a short circuit, which leads to a large potential difference across a low resistance. The result is the potentiometer heating up rapidly, producing a visible quantity of smoke and ceasing to function. To prevent problems from occurring, you may wish to inspect your students’ circuits before allowing them to connect the battery. You may also wish to give you students extra assistance by labeling the terminals of the potentiometer and the LED with tape or by color-coding them with paint or nail polish to indicate where they should be connected. Error enters this experiment with the students’ ability to see the point when the LED just begins to glow. The most difficult LED to see is the IR, because of using the camera. If the other 3 LED values give a straight line, this value might be one to eliminate. Ambient light can also affect the experiment. LEDs do not emit a single frequency of light. Instead they emit a narrow spectrum with a width of about 60 nm. The frequency values plotted on the graph are the central frequency, but when the LEDs just begin to glow, typically lower frequencies are seen. The wavelength values given for LEDs are for the LEDs provided you today. These values can vary depending on the LED. Check with the manufacturer for the correct wavelengths if you use different LEDs. I plotted the wavelengths given and got about a 20% error. I plotted the wavelengths given minus 30 nm and got about a 7% error. I plotted the wavelengths given minus 60 nm and got less than 1% error. The reason the graph will not pass through the origin is that the equation E = hf is approximate. In reality the energy is less than hf because the electrons have some thermal energy. This energy is significantly less than qV, so this fact does not account for the entire deviation from the origin. For information on the physics behind LED’s and other helpful information see: http://www.perimeterinstitute.ca/Outreach/Plancks_Constant/Measuring_Planck%27s_Constant %3A_The_Physics_Behind_LEDs/ http://www.perimeterinstitute.ca/Outreach/Plancks_Constant/Measuring_Planck%27s_Constant %3A_Appendix/