Section 12
advertisement

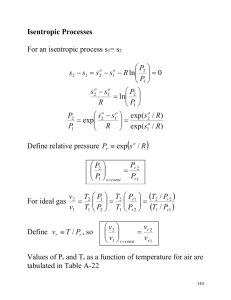
SECTION XII – ENTROPY CHANGE FOR IDEAL GASES (Exact treatment) dh dP v T T 2 2 2 dP v R dT ds c p R For an ideal gas, dh c p dT, 1 1 1 P T P T Recall Gibb’s eqn.: ds 2 s 2 s1 c p 1 P dT Rn 2 T P1 dT , which depends upon temperature alone. T Note that s is zero at absolute temperature of zero. T Define an entropy function s c p 0 2 c 1 p P dT 2 dT 1 dT c p cp s 2 s1 s 02 s10 Rn 2 0 T 0 T T P1 Unlike the change in internal energy and the change in enthalpy, the change in entropy of an ideal gas does not depend upon the temperature alone. Gibbs eqns.: du P ds dv T T ds (1) dh v dP T T For ideal gases: Pv = RT, du = cvdT, dh = cpdT From (1) ds c v dT dv R T v and from (2) ds c P dT dP R T P For constant cv, (3) gives: s = cvnT + Rnv + s1 (2) (3) ( 4) (5) integration constant and for constant cp, (4) gives: s = cpnT - RnP + s2 For constant v, (5) becomes s c v nT c1 (7) where c1 = s1 + Rnv and for constant p, (6) becomes s c p nT c 2 (6) (8) where c2 = s2 -RnP From (7) T e ( s c1 ) / c v and from (8) T e (s c 2 ) / c P constant Section XII – Entropy Change for Ideal Gases (Exact treatment) Page 53 constant pressure constant volume T s Cv Cp n=k n = (v = const) T n = 0 (P = const) n=1 s Isentropic Processes for ideal gases Isentropic constant entropy An internally reversible, adiabatic process is an isentropic process but an isentropic process is not necessarily reversible and adiabatic. Consider, for example, an isentropic process involving an incompressible substance. 2 P P dT Recall s 2 s1 c p Rn 2 s 2 s1 Rn 2 1 T P1 P1 For an isentropic process from (1) T1 = 0 K, P1 = P0 Section XII – Entropy Change for Ideal Gases (Exact treatment) to s P Pr Pr e R P0 s const relative (2) P2 = P 0 s Rn Page 54 P s P n P0 P0 R s s(T) Pr Pr (T) For an isentropic process between any two states (1) and (2) P2 P1 P P 2 0 s const P0 P1 Pr 2 s const Pr1 isentropic ( s 2 s1 ) R pressure e ratio v Relative specific volume: v r v 0 s const v0 = v at T0 = 0 K and P = P0 RT P0 1 T with Pv RT v r P RT0 s const Pr T0 s const but vr will be infinite since T0 = 0 K v Hence define v r v ref T P v r ref s const P Tref T Pref 1 T v r vT c Pr Tref P0 Pr s const T Pref P0 T P P s const 0 s const ref C: arbitrary constant (Pref and Tref are fixed) Pr Pr (T) v r v r (T) v2 v1 T Pr T P P RT v vr P 2 1 2 1 0 2 1 2 2 RT1 s const T1 P0 P2 s const T1 Pr2 s const P2 v1 v r1 s const For ideal gases with constant specific heats Recall T v s 2 s1 c v ve n 2 Rn 2 , T1 v1 T P s 2 s1 c Pve n 2 Rn 2 T1 P1 – for an isentropic process from (1) to (2), s2 = s1 c vave T2 v n 2 n R v1 s const T1 s const Section XII – Entropy Change for Ideal Gases (Exact treatment) C v ave R v2 T2 v1 s const T1 P n 2 P1 and v2 v1 v1 v2 C v ave R s const k ave k T T 2 2 s const T1 s const T1 1 T k ave 1 2 s const T1 s const R cP cv k cp c v 1 k 1 ave T 1 T2 cP T ave n 2 R s const T1 Page 55 ave 1 k ave P2 v 1 P1 s const v 2 s const T2 v 1 T1 s const v 2 k ave 5.44 Nitrogen at 800 K, 2 MPa proceeds along an isentropic path until its temperature is reduced to 300 K. Assuming ideal-gas behavior, calculate the pressure at the final state for the following conditions: (a) The nitrogen has variable specific heats. (b) The nitrogen has constant specific heats. ( s 2 s1 ) P P R (a) s 2 s1 s 2 s1 Rn 2 Process is isentropic s 2 s1 2 e P1 P1 s 2 191.682 kJ / kg mol K s 2 6.8426 kJ / kg K s1 220.907 kJ / kg mol K s 7.8859 kJ / kg K 1 P2 P1 e ( s 2 s1 ) R P2 59.5 KPa 2(e 3.5152 ) 0.0595 MPa R N 2 0.2968 kJ / kg K M N 2 28.013 kg / kg mol Section XII – Entropy Change for Ideal Gases (Exact treatment) P (b) 2 P1 T2 T1 T P2 P1 2 T1 k k 1 k k 1 , Tave 300 2 800 800 300 550 K 2 1.388 0.388 Page 56 kave = 1.388 0.0599 MPa P2 59.9 KPa 5.31 Carbon dioxide changes state from 150 kPa, 30C to 300 kPa, 300C. Calculate the entropy change of the CO2 during the process, assuming the following: (a) The CO2 is an ideal gas with constant specific heats. (b) The CO2 is an ideal gas with variable specific heats. T P (a) s 2 s1 C Pave n 2 Rn 2 T1 P1 Tave T1 T2 303 573 438 K 2 2 C Pave 0.939 (0.978 0.939)( 438 400) 0.970 kJ / kg K (450 400) (see Table D-8, p. 723) R CO 2 0.1889 kJ / kg K 573 300 s 2 s1 0.970 n 0.1889 n 0.487 kJ / kg K 393 150 P (b) s 2 s1 s 2 s1 Rn 2 P1 (215.146 213.915)(303 300) 214.284 kJ / kg mol K s1 213.915 (310 300) (241.602 240.789)(573 570) 241.033 kJ / kg mol K s 2 240.789 (580 570) M CO 2 44.011 kg / kg mol s1 214.282 241.033 4.869 kJ / kg K , s 2 5.477 kJ / kg K 44.011 44.011 Section XII – Entropy Change for Ideal Gases (Exact treatment) Page 57 300 s 2 s1 (5.477 4.869) 0.1889 n 0.477 kJ / kg K 150 5.53 Air enters an air compressor at 27C, 1 atm pressure. The air follows a reversible adiabatic process in the compressor and leaves a 2 MPa. The mass flow rate of the air through the compressor is 2.5 kg/s. Neglecting changes in kinetic energy, calculate the power required by the compressor and the temperature of the air as it leaves the compressor. Ti 300 K Tcrit air 133 K, Pi 101.3 KPa Pcrit air 3.76 MPa P e 2 MPa P crit air 3.76 MPa air can be treated as ideal Process is reversible and adiabatic Process is isentropic se = si Process in T-S diagram Pe = 2 MPa Te e e Pi = 101.3 kPa Ti ie Exit temperatrue P s e s i s e s i R n e Pi P s e s i R n e Pi 2000 6.7018 0.287 n 7.5579 kJ / kg K 101.3 Te 690 7.5579 7.5572 Te 690.45 K 700 690 7.5726 7.5572 Assume flow through compressor is steady and uniformly distributed at exit and inlet Neglect changes in potential and kinetic energy of air. These will be small compared to the change in enthalpy Section XII – Entropy Change for Ideal Gases (Exact treatment) Page 58 Conservation of energy applied to the compressor as an open system: W m m (h e h i ) W (h i h e ) Q ie ie ie adiabatic process h i 300.19 kJ / kg h e 702.52 7.5579 7.5572 h e 703.01kJ / kg 713.27 702.52 7.5726 7.5572 2.5 (300.19 703.01) 1007.1 KW W ie Power input system boundary ei e e Air, at a temperature of 155.5C and at a pressure of 0.1 MPa, undergoes an isothermal, internally reversible process. The final specific volume is 0.28 m3/kg. Find the change in entropy, heat transferred and work done Air can be treated as ideal since T1 = T2 = 428.5 K > Tcrit ave 133 K and P1 0.1MPa Pcrit ave 3.76 MPa Initial specific volume: v1 Final pressure: P2 RT1 0.287 428.5 1.23 m3 kg P1 100 RT2 0.287 428.5 439.2 KPa v2 0.28 Section XII – Entropy Change for Ideal Gases (Exact treatment) Page 59 Process on P – v and T – s diagrams P T P2 = 0.439 MPa P1 = 0.1 MPa T1 = T2 v s Change in entropy P s 2 s1 s 2 s1 R n 2 P1 Pr ocess is isothermal 439.2 0.287 n 0.4247 kJ / kg K 100 alternatively T v 0.28 s 2 s1 C vave n 2 R n 2 0.237 n 1.23 T1 v1 (T1 =T2) Q12 Air T1 = T2 = 155.5C = 628.4 K P1 = 0.2 MPa V2 = 0.23 m3/kg s 2 s1 0.4248 kJ / kg K system boundary Heat transfer: Process is internally reversible and isothermal q 12 T(s 2 s1 ) 428.5 (0.4247) 182.0 kJ / kg Work done: First Law: q12 = W12 + du W12 182.0 kJ / kg (du = cvdT, dT = 0) W12 Section XII – Entropy Change for Ideal Gases (Exact treatment) Page 60 5.54 An ideal gas in a closed system undergoes an internally reversible constant pressure process during which the temperature of the gas decreases as a result of heat transfer to the environment. Determine the correct response to each of the following and briefly justify your responses. (a) The entropy change of the gas is (greater than, equal to, less than) zero. (b) The entropy change of the environment is (greater than, equal to, less than) zero. (c) The total entropy change is (greater than, equal to, less than) zero. (a) Entropy change of the gas: P s 2 s1 s 2 s1 R n 2 P1 Environment Tenv (P1 = P2) ideal gas Q12 since T2 < T1 s 2 s1 s 2 - s1 0 (b) Entropy change of the environment: S env Q > 0 Tenv > 0 system boundary Q Tenv since environment receives heat, environment is a heat sink Senv 0 (c) Total entropy change Heat transfer through a finite temperature difference, from the gas to the environment, is an irreversible process. (S) total > 0 (Principle of increase in entropy)