A Vignette (PPTX Format)
advertisement

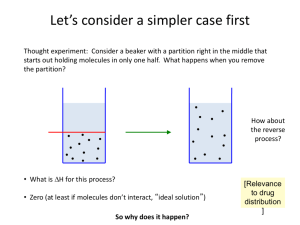
S = k ln W A vignette…. Let’s consider a simpler case first Thought experiment: Consider a beaker with a partition right in the middle that starts out holding molecules in only one half. What happens when you remove the partition? How about the reverse process? • What is DH for this process? • Zero (at least if molecules don’t interact, “ideal solution”) So why does it happen? [Relevance to drug distribution ] How about the reverse process? Thought experiment: Molecules spontaneously moving to only one half of beaker? • DH is still zero • This seems unlikely, except maybe if there are very few particles • Let’s calculate the probabilities quantitatively Probability of being on one side 1 mol N=1 p = 1/2 N=2 p = 1/4 N p = (1/2)N N ~ 6 x 1023 p = (1/2)N = very very small This fits with our intuition (and the second law) which says that things tend toward disorder, i.e., higher entropy [unless there is an input of energy to counteract …] Statistical thermodynamics tells us how to compute entropy • There are multiple ways to define entropy, including the one on Boltzmanns tombstone In modern notation, this is typically written S = kB ln W W is the number of configurations available to a system, and is related to the probabilities we just calculated. Note that S is a state function, and positive values of DS are favorable, not negative like DH. Units on S are J/K (more on this later). Let’s compute DS for this process This is not just a thought experiment. This is equivalent to entropy change upon dilution, or mixing two solutions. State 1 State 2 S1 = kB ln W1 S2 = kB ln W2 We can define the number of “configurations” of the system in different ways, but regardless, W clearly doubles for a single molecule when we double the volume. N=1 DS = kB ln 2 = 1.38x10-23 J/K * 0.693 ~ 10-23 J/K To get units of energy, multiply by temperature (300 K), which gives 3x10-21 J. TINY amount of energy. We assume that the 2 molecules are uncorrelated, i.e., the configuration of one is unrelated to the other. (This is again the definition of an “ideal solution”.) N=1 DS = kB ln 2 N=2 DS = kB ln 4 This then implies that the total number of configurations is the product (not sum) of the configurations for each molecule. N=1 DS = kB ln 2 N=2 DS = kB ln 4 N molecules DS = kB ln 2N =NkBln 2 N=1 DS = kB ln 2 N=2 DS = kB ln 4 N = NA = 1 mole DS = NAkBln 2 = R ln 2 N = # of molecules NA = Avogadro’s number kB = Boltzmann’s constant R = NAkB = gas constant N=1 DS = kB ln 2 N=2 DS = kB ln 4 Generalizing to any volume change, Molar entropy: æ V2 ö DS = R ln ç ÷ è V1 ø N = NA = 1 mole DS = NAkBln 2 = R ln 2 N = # of molecules NA = Avogadro’s number kB = Boltzmann’s constant R = NAkB = gas constant We can also think of this in terms of concentrations State 1 æn ö æ V2 ö æ M1 ö M2 ÷ ç DS = R ln ç ÷ = R ln ç = R ln ç ÷ ÷ n ç ÷ è V1 ø è M2 ø M è 1ø State 2 Convert to concentrations, in molarity units: M = n/V, so V = n/M V = volume N = # of molecules n = # of moles R = NAkB = gas constant There are other flavors of entropy • We focused here on “translational” entropy, which is equivalent to entropy of dilution, or entropy of mixing • There is also “rotational” entropy and “vibrational entropy”, very important in drug binding as well Free Energy • At constant pressure, the correct criterion is DG < 0 where G = H – TS • If we additionally assume constant T, you get the famous equation DG = DH – TDS • Key point: note the sign on the entropy term • When DG = 0, this is called “equilibrium” • Molar free energy for a substance also referred to as “chemical potential”: An intuitive feel for free energy? • Enthalpy = energy • Entropy = disorder • Free energy = ?? • Best understood as a statement of the 2nd law of thermodynamics Free energy of dilution/mixing State 1 Recall that … State 2 DH = 0 æ V2 ö æ M1 ö DS = R ln ç ÷ = R ln ç ÷ è V1 ø è M2 ø æ V2 ö æ M1 ö æ M2 ö DG = -RT ln ç ÷ = -RT ln ç ÷ = RT ln ç ÷ è V1 ø è M2 ø è M1 ø Derivation of DG=-RTln K for a simple AB reaction At equilibrium (constant T, P): A B Assume ideal solution, MA mA (aq) = m (aq) + RT ln M° 0 A Mº = 1 molar (to make argument of the logarithm unitless) MB mB (aq) = m (aq) + RT ln M° 0 B Equating these two: MA MB 0 m (aq) + RT ln = mB (aq) + RT ln M° M° 0 A MA MB 0 m (aq) + RT ln = mB (aq) + RT ln M° M° MA MB 0 0 mB (aq) - mA (aq) = RT ln - RT ln M° M° 0 A Constant that depends only on nature of reactants and products DEFINE KM= (MB/MA)eq Extension to more general equilibria: aA + bB cC + dD At equilibrium (constant T, P; closed system): c C d D a A b B æM M ö KM = ç ÷ è M M øeq c C a A d D b B Alternate Notation KC [C ]c [ D ]d [ A ] a [ B ]b eq Other concentration units Derivations are extremely similar. For molality (mol/kg solvent), the chemical potential can be written as mi mi (aq) = m (aq) + RT ln m° o i where the standard state is now defined by mº = 1 molal. The equilibrium constant winds up looking like D G -RT ln K m 0 Km mB mA Expression for gasses is also very similar, with partial pressures replacing concentrations.