5 Entropy of Ideal Gas
advertisement
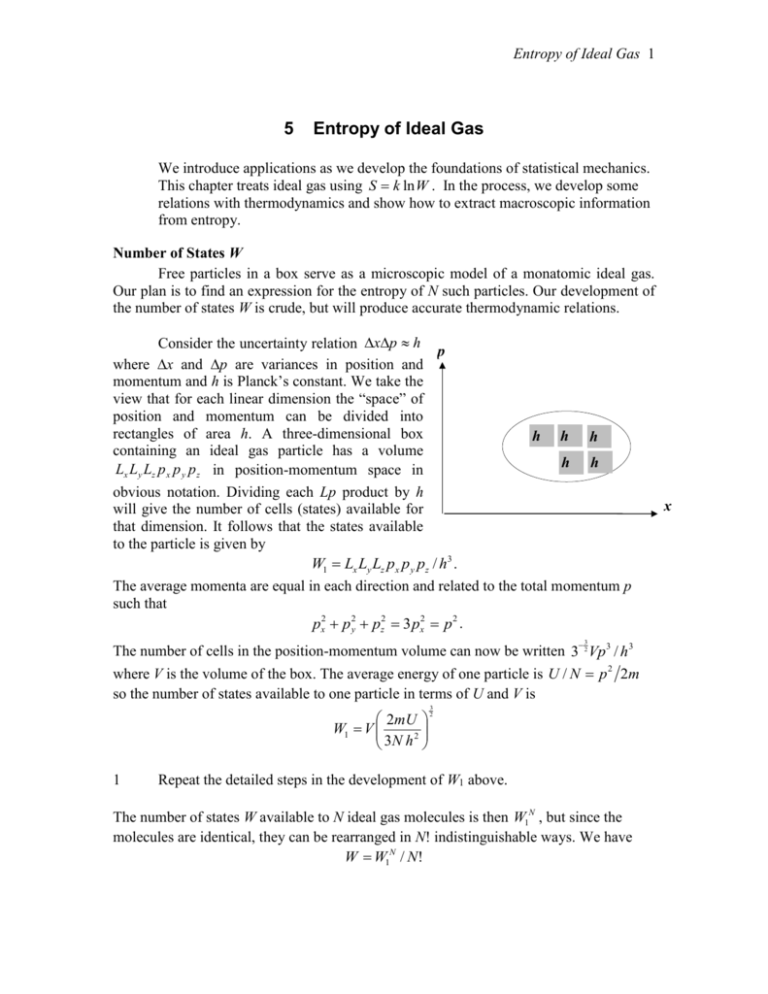
Entropy of Ideal Gas 1 5 Entropy of Ideal Gas We introduce applications as we develop the foundations of statistical mechanics. This chapter treats ideal gas using S k ln W . In the process, we develop some relations with thermodynamics and show how to extract macroscopic information from entropy. Number of States W Free particles in a box serve as a microscopic model of a monatomic ideal gas. Our plan is to find an expression for the entropy of N such particles. Our development of the number of states W is crude, but will produce accurate thermodynamic relations. Consider the uncertainty relation xp h p where x and p are variances in position and momentum and h is Planck’s constant. We take the view that for each linear dimension the “space” of position and momentum can be divided into rectangles of area h. A three-dimensional box h h h containing an ideal gas particle has a volume h h Lx Ly Lz p x p y p z in position-momentum space in obvious notation. Dividing each Lp product by h will give the number of cells (states) available for that dimension. It follows that the states available to the particle is given by W1 Lx Ly Lz px p y pz / h3 . The average momenta are equal in each direction and related to the total momentum p such that px2 p 2y pz2 3 px2 p 2 . 3 The number of cells in the position-momentum volume can now be written 3 2 Vp3 / h 3 where V is the volume of the box. The average energy of one particle is U / N p 2 2m so the number of states available to one particle in terms of U and V is 3 2mU 2 W1 V 2 3N h 1 Repeat the detailed steps in the development of W1 above. The number of states W available to N ideal gas molecules is then W1N , but since the molecules are identical, they can be rearranged in N! indistinguishable ways. We have W W1N / N! x Entropy of Ideal Gas 2 2 Show that the entropy of a monatomic ideal gas according to the W of problem 1 is 3 2mU 2 Nk ln N S Nk ln V 2 3N h where N! is approximated by the leading term of Sterling’s formula. Applications to Macro Variables: Thermodynamics Now that an expression for entropy is known, all macroscopic properties of the ideal gas can be found. Substitute dW PdV into dU TdS dW , dU TdS PdV (1) The negative sign on the work term is appropriate for the gas applying pressure to the vessel. We will see that once S is known as a function of U and V, all macro-state equations can be derived using Eq.(1). 3 Solve Eq.(1) for dS and compare the result with the mathematical identity, S S dS (U ,V ) dU dV U V V U 1 P S S Show (many books define temperature with this) and U V T V U T 4 Substitute S from problem 2 into the expressions from problem 3 to show 3 U NkT and PV NkT 2 These results are the state equations for monatomic ideal gas. They represent the macroscopic properties of the gas. Much of the literature, especially in chemistry, describes quantities in moles where one mole is NA=6.031023 particles. Rather than Boltzmann’s constant k, these authors use the gas constant R where N Ak R 5 Let n represent the number of moles in N particles, N nN A and rewrite the state equations of problem 4 replacing N and k with n and R. Show the heat capacity 3 ( C U T ) is C nR . 2 Adiabatic Processes Often, systems are either insulated from heat transfer or a process takes place too quickly for a significant heat transfer to occur. These are adiabatic processes and we characterize them with the condition dS 0 or S = constant. Entropy of Ideal Gas 3 6 For the entropy of problem 2 require that S = constant while N is fixed. Show that 2 2 2 TV 3 constant (or equivalently, T1V1 3 T2V2 3 ). 7 A Helium balloon at 310 K rises in the atmosphere and swells in the reduced pressure increasing its volume by 10%. Assume the process is adiabatic and find the new temperature. [ans 291 K] 8 (a) Using the method of this chapter, calculate an expression for the entropy of an ideal gas constrained to a two-dimensional area A. AmU AmU kN ln N Nk ln 2 2 ] [ans. S Nk ln 2 Nh N h (b) Find the heat capacity of this gas in terms of number of moles and the gas constant. [ans. C nR ] (c) Show that an adiabatic condition for this gas is T1 A1 T2 A2 . Summary This chapter illustrated some of the predictive power that follows from knowing the form of entropy. Later chapters will introduce more systematic ways of calculating entropy, energy, and other thermodynamic functions.











