Microcanonical Ensemble: Probability & Entropy
advertisement

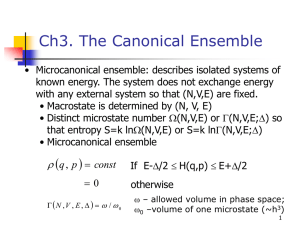
The microcanonical ensemble Finding the probability distribution We consider an isolated system in the sense that the energy is a constant of motion. N E H ( p, q) E We are not able to derive from first principles Two typical alternative approaches Postulate of Equal a Priori Probability Construct entropy expression from it and show that the result is consistent with thermodynamics Use (information) entropy as starting concept Derive from maximum entropy principle Information entropy* *Introduced by Shannon. See textbook and E.T. Jaynes, Phys. Rev. 106, 620 (1957) Idea: Find a constructive least biased criterion for setting up probability distributions on the basis of only partial knowledge (missing information) What do we mean by that? Let’s start with an old mathematical problem Consider a quantity x with discrete random values x1 , Assume we do not know the probabilities x2 , ..., xn p1 , p2 , ..., pn We have some information however, namely p n i 1 i 1 and we know the average of the function f(x) (we will also consider cases where we know more than one average) n f ( x ) pi f ( xi ) i 1 With this information, can we calculate an average of the function g(x) ? To do so we need all the n p i 1 i 1 and n p1, p2 , ..., pn but we have only the 2 equations f ( x ) pi f ( xi ) i 1 we are lacking (n-2) additional equations What can we do with this underdetermined problem? There may be more than one probability distribution creating We want the one which requires no further assumptions We do not want to “prefer” any pi if there is no reason to do so n f ( x ) pi f ( xi ) i 1 Information theory tells us how to find this unbiased distribution (we call the probabilities now rather than p) Shannon defined information entropy, Si: Si k n lnn n n or Si k dpdq ( p, q )ln ( p, q ) for continuous distribution with normalization n 1 dpdq ( p, q) 1 We make plausible that: - Si is a measure of our ignorance of the microstate of the system. More quantitatively - Si is a measure of the width of the distribution of the n. Let’s consider an extreme case: An experiment with N potential outcomes (such as rolling dice) However: Outcome 1 has the probability 1=1 Si k n lnn 0 Outcome 2,3,… ,N have n=0 n -Our ignorance regarding the outcome of the experiment is zero. -We know precisely what will happen - the probability distribution is sharp (a delta peak) Let’s make the distribution broader: Outcome 1 has the probability 1=1/2 Outcome 2 has the probability 2=1/2 Outcome 3,4, … ,N have n=0 Let’s consider the more general case: Outcome 1 has the probability 1=1/M Outcome 2 has the probability 2=1/M . . . Outcome M has the probability M=1/M Outcome M+1, … ,N have n=0 1 1 1 1 Si k n lnn k ln ln 0 ... 0 2 2 2 2 n 1 1 k ln 2 ln 2 k ln 2 2 2 Si k n lnn n 1 1 1 1 1 1 k ln ln ... ln ... 0 M M M M M M k ln M So far our considerations suggest: Ignorance/information entropy increases with increasing width of the distribution Which distribution brings information entropy to a maximum For simplicity let’s start with an experiment with 2 outcomes Binary distribution with 1, 2 and 1+ 2=1 Si k 1ln1 2ln2 1 2 1 with Si k 1ln1 1 1 ln 1 1 dSi 1 1 k ln1 1 ln 1 1 1 1 k ln d i 1 1 1 1 0.7 0.6 maximum 0.5 dSi k ln 1 0 d i 1 1 1 1 1 1 Si 0.4 1 1/ 2 2 uniform distribution 0.3 0.2 0.1 0.0 0.0 0.2 0.4 0.6 0.8 1 1.0 Lagrange multiplier technique Once again Si k 1ln1 2ln2 at maximum with 1 2 1 constraint F (1, 2 , ) k 1ln1 2ln2 1 2 1 F k ln 1 1 0 1 F k ln 2 1 0 2 F 1 2 1 0 From http://en.wikipedia.org/wiki/File:LagrangeMultipliers2D.svg Finding an extremum of f(x,y) under the constraint g(x,y)=c. 1 2 1 2 1 1 1/ 2 2 uniform distribution Let’s use Lagrange multiplier technique to find distribution that maximizes M Si k n lnn n 1 M F ( 1 , 2 ,..., M , ) k n lnn n 1 n 1 n1 M F k ln j 1 0 j with M n 1 Si n max 1 1 2 ... M e1 / k 1 2 ... M 1 M uniform distribution maximizes entropy k ln M In a microcanonical ensemble where each system has N particles, volume V and fixed energy between E and E+ the entropy is at maximum in equilibrium. Distribution function - When identifying information entropy with thermodynamic entropy 1 const . Z (E) ( p, q) 0 otherwise if E H ( p, q) E Where Z(E) = # of microstate with energy in [E,E+ ] called the partition function of the microcanonical ensemble Information entropy and thermodynamic entropy When identifying k=kB S kB n lnn n has all the properties we expect from the thermodynamic entropy (for details see textbook) We show here S is additive S 1 2 S 1 S 2 S1 (1) n : probability distribution for system 1 S2 (2) m : probability distribution for system 2 Statistically independence of system 1 and 2 probability of finding system 1 in state n and system 2 in state m S (1 2) k B n(1) m(2) ln n(1) m(2) k B n(1) m(2) ln n(1) ln m(2) n,m n,m k B m(2) n(1) ln n(1) k B n(1) m(2) ln m(2) m n n m k B n(1) ln n(1) k B m(2) ln m(2) S (1) S (2) n m n(1) m(2) Relation between entropy and the partition function Z(E) S kB n lnn n 1 1 k B ln Z (E) n Z (E) kBlnZ ( E ) n 1 Z (E) 1 S kBlnZ (E) Derivation of Thermodynamics in the microcanonical Ensemble Where is the temperature ? In the microcanonical ensemble the energy, E, is fixed E (S ,V ) U ( S ,V ) with dU TdS PdV 1 S T U V and P S T V U Let’s derive the ideal gas equation of state from the microcanonical ensemble despite the fact that there are easier ways to do so Major task: find Z(U) :=# states in energy shell [U,U+ ] Z (U ) p U+ 1 3N 3N d p d q 3N N ! h U H ( p ,q )U U q another leftover from qm: phase space quantization, makes Z a dimensionless # “correct Boltzmann counting” Solves Gibb’s paradox requires qm origin of indistinguishability of atoms We derive it when discussing the classical limit of qm gas U N pi2 2 m U i 1 d 3N VN pd q N ! h3 N 3N U N pi2 2 m U i 1 pi2 i 1 2m H For a gas of N non-interacting particles we have 1 Z (U ) N ! h3 N N d 3 p1 d 3 p2 ...d 3 pN N pi2 3N dim. sphere in momentum space 2 mU i 1 2 m (U ) 2mU VN Z (U ) N ! h3 N U N 3 3 3 d p1 d p2 ...d pN pi2 2 m U V N C3 N N ! h3 N 2m(U ) 3N / 2 2mU 3N / 2 V2 dim S 2dim dr 2 rdr r 2 i 1 V3N ( pU 2m(U )) V3N ( pU 2mU ) Remember: 4 V3 dim S3dim dr 4 r 2 dr r 3 3 ... V3 N dim S3 N dim dr r 3 N 1dr C3 N r 3 N 3N / 2 U 3 N / 2 N V U 1 const U S kBlnZ (U ) U 3 N / 2 3 kB N ln V N ln U ln 1 ln const 2 U In the thermodynamic limit of N V U lim a n 0 n N const V for 0 a 1 ln1=0 http://en.wikipedia.org/wiki/Exponentiation 3 S k B N ln V N ln U 2 U 3 N / 2 ln 1 U ln const 3 S kB N ln V N ln U ln const 2 1 S with T U V P S T V U U 3 Nk BT 2 PV NkBT