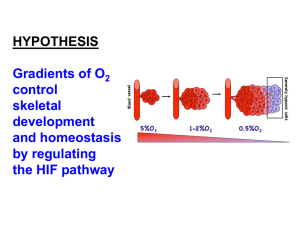
EXPERIMENTAL PROCEDURES
advertisement

EXPERIMENTAL PROCEDURES Cell Lines and Reagents. U251 human glioma cells were maintained as described previously (Rapisarda et al. 2002). HCT116 cells were maintained routinely in RPMI medium supplemented with 5% heat-inactivated fetal bovine serum, penicillin (50 IU/ml), streptomycin (50 µg/ml), and 2 mM glutamine. Inducible ATR kinase dead (ATRkd) stably transfected transfected cells (22.4.I.853) were cultured in the presence or absence of doxacycline (1g/ml) for 48 hours prior to the beginning of the experiment. Cells were maintained at 37°C in a humidified incubator containing 21% O2 and 5% CO2 in air (referred to as normoxic conditions). Hypoxia treatment was performed in an Invivo2 400 hypoxic workstation (Ruskin Technologies, OH) at 1% O2 (hypoxia). Immunoblot Analysis. Whole cell lysate was prepared as described previously (Rapisarda et al. 2004d). Data presented are representative of at least three independent experiments performed. Real-Time PCR - Total RNA extraction and Reverse transcription-PCR was performed as described previously (Rapisarda et al. 2002). To measure human VEGF and HIF-1 expression, real-time PCR was performed using an ABI-Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA) as described previously (Rapisarda et al. 2002). Primers and specific probes were obtained from Applied Biosystems. Human VEGF and HIF-1 primers and probes were described previously (Rapisarda et al. 2004b). Used as an internal control, 18S rRNA was assessed using premixed reagents from Applied Biosystems. Results are expressed as fold changes relative to mRNA levels of untreated cells in normoxic conditions (equal to 1) and are the average of three independent experiments. HIF-1 Protein Translation Assay. U251 cells were exposed to methionine/cysteine-free RPMI 1640 for 4 h after being exposed to UVC 20-200J/m2. Cells then were labeled by incubation with methionine/cysteine-free medium containing 35S-methionine/cysteine (Trans Label; ICN Biomedicals) at a final concentration of 150 µCi/ml at 37°C for the indicated time. Radiolabel incorporation was then monitored by both size fractionation of lysates (using a 4-20% Tris-Glycine gel and then autoradiography) and trichloroacetic acid (TCA) precipitation.