Synthesis of 2
advertisement

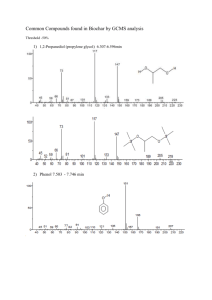
Synthesis of 2-methylpropyl propanoate by Fischer Esterification Hannah Chick, Eli Levine, Molly McMahon Abstract 2-methylpropyl propanoate (3) was synthesized, via Fischer esterification, from propanoic acid (1) and 2-methylpropan-1-ol (2). The product was confirmed using 1HNMR and IR spectroscopy. A yield of 37.3% was obtained. Introduction The purpose of this exercise was to synthesize ester 3. In the process, we became familiar with common laboratory techniques and esterification reactions. Ester 3, a compound that simulates the odor of rum, was chosen to be synthesized following the procedure outlined in Stocksdale (2002). Ester 3 was synthesized using a reflux reaction of compounds 1 and 2 and isolated via fractional distillation. The structure of ester 3 was confirmed using IR and 1H-NMR spectroscopy. O O + OH propanoic acid (1) O OH 2-methylpropan-1-ol (2) 2-methylpropyl propanoate (3) Figure 1. Reaction for synthesis of 2-methylpropyl propanoate (3) by Fischer esterification Results and Discussion Based on IR and 1H-NMR spectroscopic data, the product of the synthesis was determined to be ester 3. There were significant IR spectrum peaks at 2965, 1741, and 1192 cm-1 representing, respectively, sp3 C-H, carbonyl group, and C-O stretches. (a) (a) H H (a) H (d) O (c) (c) H H H O H H (b) (b) H (e) H (e) H (e) H H H (e) (e) (e) Figure2. Structure of ester 3 illustrating unique hydrogen groups seen in 1H-NMR spectrum. An 1H-NMR spectrum showed a doublet integrated to six hydrogens at 0.893ppm due to the hydrogens (e) of two terminal methyl groups. A triplet1, integrated to three hydrogens (a), appeared at 1.159ppm due to the third terminal methyl group. A multiplet, that integrated to 1 hydrogen (d), was shown at 1.728ppm. A 2H (b) quartet appeared at 2.415ppm. Finally, there was a 2H (c) doublet at 3.824ppm. The higher shifts of groups b and c were due to the proximity of the attached carbons to the carbonyl and ketone groups. The product yield was determined to be 37.3% and based on the spectra obtained the product appeared to be pure. The yield was reduced as the result of a spill that occurred prior to distillation. 1 One peak of this triplet overlapped with the doublet at .893ppm. Based on the Aldrich catalogue information for butyl propionate the boiling point for isobutyl propionate was estimated to be between 130 and 150o C. The actual boiling point of the product was measured at 125o C. It must be noted, however, that there was a low pressure front moving through the area at the time of distillation. This may have had an impact on the measured boiling point of the product. Experimental Section General Procedures. All the materials used in this procedure were obtained from the Earlham College organic chemistry laboratory. The experiment followed the general steps outlined in Stocksdale (2002). Synthesis of Ester 3. 5 mL (0.067 mol) of propanoic acid was mixed with 6.83 mL (0.074 mol) of 2-methylpropan-1-ol and 1 drop H2SO4. The mixture was heated to reflux in a “Dean-Stark”-like apparatus until no more condensation accumulated. The mixture was removed from the heat to cool. Once cooled, the liquid was removed from the Claisen head with a pipette and transferred to a test tube. The organic layer was removed and placed in a 25 mL Erlenmeyer flask. Approximately 200 mg of NaCO3 was added to the liquid that had been collected from the Claisen head. The un-refluxed mixture was then added. The sample was then dried using anhydrous NaSO4. Distillation of Ester 3. Two Erlenmeyer flasks were weighed and their masses recorded. A distillation apparatus was set up and the distillation process was monitored to determine the boiling points of the distillates. IR and 1H-NMR spectra were obtained for the two distillates obtained. The product yield was 37.3%. Spectra of Ester 3. IR (neat): 2965, 1741, 1192 cm-1. 1H-NMR (CDCl3, 60MHz); .893 (d, 6H), 1.159 (t, 3H), 1.728 (m, 1H), 2.415 (q, 2H), 3.824 (d, 2H). Literature Cited Stocksdale, Mark. Organic Lab Projects Lab Manual Experiment #4. 2002. Acknowledgments A big, rousing thanks to you, Mark “the human fly-swatter” Stocksdale, for a fabulous lab. Kudos to the two lab TA’s, Hillary and Lauren, for a job well done.