Reactions of Enols and Enolates
advertisement
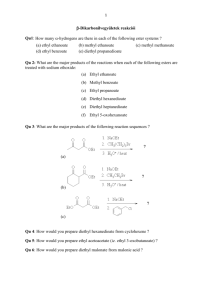
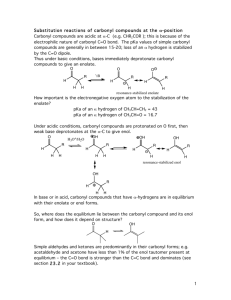
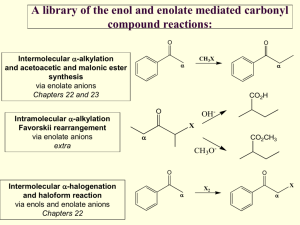
Reactions of Enols and Enolates Ketones and aldehydes are in equilibrium with a small amount of the corresponding enol. This can be problematic, if one desires to produce a stereogenic center a to a C=O. But the enol form is also useful since the electron-rich double bond can serve as a mild nucleophile toward reactive electrophiles. Alternatively, the proton on the a-carbon can be abstracted by a strong base to generate a (resonance-stabilized) enolate anion. This enolate anion is a much stronger nucleophile than the corresponding enol. The Aldol Condensation Reaction Ketone Enolate + Aldehyde Notice that, in this case, the product alcohol dehydrated to form an a,b-unsaturated carbonyl as a product. Intramolecular Aldol Ester Enolate + Aldehyde Ester Enolate + Ester = Claisen Condensation Intramolecular Claisen Condensation = Dieckmann Condensation 1,4-Conjugate Addition: The Michael Reaction (Notice that the presence of the conjugated C=C-C=O system is crucial, since this both polarizes the C=C and also leads to a resonance-stabilized carbanion, after addition of the nucleophile) Conjugate additions are promoted by the addition of copper salts, which are believed to function by coordination with the C=C, prior to addition of the nucleophile. Decarboxylation Reactions Decarboxylation produce CO2, and are thus thermodynamically favorable. These reactions proceed most readily when the COOH moiety is attached to an electronegative atom, like oxygen or nitrogen. Unstabilized Carbanion is NOT formed However, even in the case of simple carboxylic acid, decarboxylation may proceed readily, if the resultant carbanion is stabilized by resonance, or other electronegative substituent, as shown for acetoacetic acid below. Stabilized (enolate) carbanion is formed The reaction probably proceeds through a cyclic transition state as shown below. Notice that saponification of the ester moiety (followed by acidification) does not produce the corresponding carboxylic acid, but instead the decarboxylated product. (Note that the ketoester is stable, but the corresponding ketoacid is not.) Carbamates are frequently used as ‘protecting groups’ for amines. Recall that tert-butyl esters are cleaved by strong acid, since the tertiary carbocation is the most stable. In the example below, cleavage of the ester is followed by decarboxylation. The tert-butyloxycarbonyl group is often used as a protecting group for amines, and is abbreviate Boc. The commercially available reagent, di-tert-butyldicarbonate, (Boc2O), is used to form the Boc-protected amine, which is then manipulated synthetically as desired, and eventually cleaved with strong acid as shown above. Recall that benzyl esters are cleaved by hydrogenolysis. Notice that, in the example below, the ester is cleaved, followed by decarboxylation to produce the free amine. (Notice that the tert-butyl ester and the ethyl carbamate are unaffected by these conditions) The carbobenzyloxycarbonyl protecting group, shown previously, is abbreviated Cbz, or sometimes Z. The Acetoacetic Ester Synthesis The Acetoacetic Ester Synthesis uses an extra ethoxycarbonyl group to stabilize the anion alpha to the ketone. Following alkylation of the (highly stabilized) enolate, the carboethoxy group is removed by hydrolysis and decarboxylation. The Malonic Ester Synthesis