Hydrogen Production by Natural Gas Assisted Steam Electrolysis

CACHE Modules on Energy in the Curriculum
Fuel Cells
Module X (Draft): H
2
Production by Natural Gas Assisted Steam Electrolysis
Module Author: Michael D. Gross
Module Affiliation: Bucknell University
Course: Membrane Separations
Text Reference: Geankoplis 4 th
ed., Chapter 13
Concept Illustrated:
Problem Motivation: Fuel cells are a promising alternative energy conversion technology. Hydrogen is currently the preferred fuel for fuel cells. The generation of pure hydrogen is important, particularly for low temperature fuel cells such as PEMFC, because the Pt catalyst is sensitive to impurities. One way to generate hydrogen is by electrolysis, a process that uses electricity to decompose water into hydrogen and oxygen.
One could accomplish this by running a fuel cell backwards as discussed in Module x.
Another route would be to supply methane at the anode and water at the cathode. This method, called Natural Gas Assisted Steam Electrolysis (NGASE), produces hydrogen without supplying electricity from an external source. In this configuration, the electrolyzer operates as a fuel cell and the electricity is generated by the electrochemical reaction.
The NGASE reactions are: Anode:
Cathode:
Overall:
CH
4
+ 4O
-2
CO
2
+ 2 H
2
O + 8 e
-
4 H
2
O + 8 e
-
4 O
-2
+ 4 H
CH
4
+ 2 H
2
O
4 H
2
2
+ CO
2 e e -
CH
4
CO
2
CH
4
CH
4
H
2
O
CO
2
CH
4
CH
4
H
2
O
O 2-
O 2-
O 2-
O
2-
H
2
H
2
O
H
2
O
H
2
H
2
H
2
H
2
O
H
2
Anode Cathode
Electrolyte
Figure 1: Reactions within NGASE
Electron
Flow
(Current)
Electric Load
Cell Voltage
CH
2
4
In In
Anode
Fuel Cell
Chamber
Cathode
Gas
Chamber
H
2
& &
H
2
O
Out
Air
Out
Figure 2: Flow Diagram for NGASE
Draft 1 - 1 - September 18, 2008
For each mole of methane consumed, 4 moles of H
2
are generated and 8 moles of electrons are passed through the external circuit. To convert electron flow (moles of electrons/s) to electrical current (coulombs/s or amps), one would use Faraday’s constant: F
96 , 485 coulombs / mole of electrons. The rate of hydrogen production is directly related to the current as follows: i
nF dN
H2 dt or dN dt
H2 i nF where i
current density
amperes cm
2 n
2 moles
1 mole of of
H e
-
2
coulombs s
cm
2
F
96,485 coulombs mole of e
dN
H2 t
moles of H
2 cm
2 s
Figure 3 shows the relationship between current density, i, and electrolyzer voltage.
There are several things to note here.
0.3
E
OCV
= 0.25 V
0.2
0.1
Draft 1
0.0
0.0
0.1
0.2
Current Density, A•cm -2
Figure 3. A performance curve for NGASE.
0.3
- 2 - September 18, 2008
The theoretical maximum voltage of this electrolyzer is 0.25 V. This is called the “open circuit voltage” E
OCV
.
Any drop in voltage from E
OCV
is termed “overvoltage.” It is desired to minimize the overvoltage so that the electrolyzer can operate as efficiently as possible.
The hydrogen reaction rate is directly proportional to the current, since for each hydrogen molecule that reacts, two electrons are formed.
For operating voltages (E op
) 0 ≤ E op
≤ E
OCV
, hydrogen is produced without an external power source. This corresponds to a possible current range of 0.25
A·cm 2 to 0 A·cm 2 .
At current densities between 0 A/cm
2
and 0.25 A/cm
2
, there is a linear fall in voltage as the current density increases. Assuming fast kinetics, this voltage drop is caused by a resistance to oxygen ion flow across the electrolyte membrane. In this case the operating voltage can be described as E op
= E
OCV
– iRA, where i is current density, R is resistance, and A is cross sectional area of the electrolyzer. In physics and electrical engineering, this effect is referred to as Ohm’s law.
The maximum rate of hydrogen generation corresponds to the maximum current, 0.25 A/cm 2 and E op
=0, which is called short circuit. It is always desired to operate at short circuit since this is the highest and most efficient rate of H
2
generation.
While the NGASE method uses an electrochemical device for separation of water into hydrogen and oxygen, it draws many parallels to typical membrane separation processes across a dense membrane. Our primary interest is the rate of H
2
production, however, O
2is the species transported across the membrane and so the flux across the membrane is written with respect to O 2. For every one mole of H
2
produced, 2 moles of e are collected and one mole of O
2-
is produced at the cathode. The flux equations for an electrolyzer and a separation with a dense membrane are compared below.
Draft 1 - 3 - September 18, 2008
c io
N i c iL
L
Figure 4. Concentration profile for a membrane process
Membrane
Separation
Table 1. Flux equations for a membrane process and NGASE.
N i
D
L i
c iO
Equations
c iL
mol O cm
2 s
2 -
Units
s cm
2
cm
mol O
2 cm
3
N
O
2
i nF
mol O
2 cm
2 s
C cm
2 s
mol O
2 mol e
-
mol e
-
C
V
i
RA V
cm
C
2 s
cm
2
C
s
NGASE i
V
RA
E
OCV
E op
E
OCV
RA
E op
L
C cm
2 s
V
1
cm
1 cm
V
cm
2 i
E
OCV
E op
L
N
O
2
L
nF
E
OCV
E op
i
N
O
2 -
flux current density
E
OCV
E op
open circuit vo operating voltage ltage
RA
area specific resistance
O 2 conductivi
L
thickness of ty
the
(S/cm membrane or 1/
cm)
D
diffusivit c iO and c iL y
concentrat ions at the membrane interfaces
mol O
2 cm
2 s
V
cm
2
mol O
2 -
mol e
mol e
-
C
Draft 1 - 4 - September 18, 2008
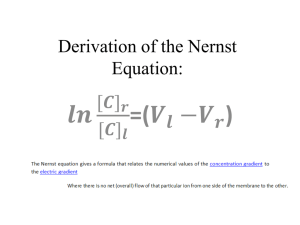
The only difference between the flux equations for the electrolyzer and membrane separation is that the electrolyzer takes into account electrical units. For the electrolyzer, voltage directly corresponds to the concentration of oxygen at each membrane interface via the Nernst equation. The concentration of oxygen is typically expressed as a partial pressure of oxygen, P
O2
. For more information regarding the Nernst equation, refer to
Module 9, under Thermodynamics.
Draft 1 - 5 - September 18, 2008
Example Problem
A company is developing a new car powered by a fuel cell system that runs on H
2
. You have been asked to consider generating the H
2
by NGASE. The H
2
tank to be used is 10 liters in volume and a fill-up requires a pressure of 500 psi. a.
The electrolyzer should be designed for maximum performance. Plot the maximum rate of H
2
generation as a function of electrolyte membrane thickness
(1 μm to 1 mm) given the following data. What is the ideal membrane thickness?
E
OCV
= 0.25 V
σ = 0.02 S/cm (S = 1/Ω) b.
Given the following data, how long would it take to fill the tank? Assume an ideal gas and 25ºC.
Electrolyzer stack consists of 100 cells in series
Each cell has an area of 10 cm
2
Electrolyte membrane thickness = 10 μm
Example Problem Solution
Part a
Step 1. Calculate the flux of O
2-
, N
O2-
, across the electrolyte membrane.
N
O
2
L
nF
E
OCV
E op
The conductivity, σ, and open circuit voltage, E
OCV
, were given in the problem statement. n
2 mol mol O e
2
-
F
96485
C mol e
-
The maximum rate of H
2
generation will occur at the maximum current density. The maximum current density corresponds to E op
= 0 V.
For an electrolyte membrane thickness of 10 μm:
Draft 1 - 6 - September 18, 2008
N
O
2
L
nF
E
OCV
E op
1
0.02
cm
10
10
4 cm
2 mol e
1 mol O
-
2 -
96485
C mol e
-
0 .
25 V 0 V
N
O
2
2 .
59
10
5 mol O
2 cm 2 s
The oxygen ion flux as a function of electrolyte membrane thickness is summarized in the following table: l (cm) N
O2-
1.00E-04
1.00E-03
1.00E-02
1.00E-01
2.59E-04
2.59E-05
2.59E-06
2.59E-07
Step 2: Calculate the rate of hydrogen generation with the oxygen flux.
Every one mole of H
2
O reacted, generates one mole of H
2
and one mole of O
2-
.
Therefore, the rate of H
2
generation is equal to the flux of oxygen ions.
1.E-03
1.E-04
1.E-05 l (cm)
1.00E-04
1.00E-03
1.00E-02
1.00E-01 dN
H2
/dt
2.59E-04
2.59E-05
2.59E-06
2.59E-07
1.E-06
1.E-07
1.E-05 1.E-04 1.E-03 1.E-02 1.E-01 1.E+00 l (cm)
Ideally, the smallest electrolyte membrane thickness possible would be used. However, in real systems the electrolyte is typically 10 μm thick or more. Membranes thinner than 10
μm can leak, causing the reactants on either side of the membrane to mix. Once reactants from either side of the electrolyte mix, the concentration of oxygen is equal on both sides and E
OCV
= 0.
Part b
From part a, we know for a 10 μm thick electrolyte membrane
Draft 1 - 7 - September 18, 2008
dN
H
2 dt
2.59E
05 mol H cm 2 s
2
Total area of electrolyzer system:
# of cells in stack
area of one cell
100 cells
10 cm
2 cell
1000 cm
2
The moles of H
2
required can be calculated with the ideal gas law, PV=nRT.
P
500 psi
1 atm
14.696
psi
34 atm n
34 atm
10 L
L
0.08206
mol atm
K
298 K
13 .
9 mol H
2 fill up time
n area
dN
H
2 dt
13.9
mol H
2
1000 cm
2
2 .
59 E
04 mol H
2 cm
2 s
540 sec onds ~ 9 min
Draft 1 - 8 - September 18, 2008
Home Problem
The example problem demonstrated how the flux across an electrolyte membrane changes with thickness. This problem addresses how the flux changes with temperature.
The conductivity of an electrolyte membrane as a function of temperature (in Kelvin) is as follows:
3.6
10 5
T
-8
10
4
e RT
A company is developing a new truck powered by a fuel cell system that runs on H
2
, and you have been asked to consider generating the H
2
by NGASE. The H
2
tank to be used is
20 liters in volume and a fill-up requires a pressure of 750 psi. a.
The electrolyzer should be designed for maximum performance. Plot the maximum rate of H
2
generation as a function of electrolyzer temperature (500ºC to 1000ºC) given the following data. What is the ideal operating temperature of the electrolyzer?
Electrolyte membrane thickness = 10 μm
E
OCV
= 0.25 V b.
Given the following data, how long would it take to fill the tank? Assume an ideal gas and a tank temperature of 25ºC.
Electrolyzer stack consists of 50 cells in series
Each cell has an area of 5 cm
2
Electrolyzer T = 800ºC
Draft 1 - 9 - September 18, 2008