Atomic Emiss LAB my version 1213
advertisement

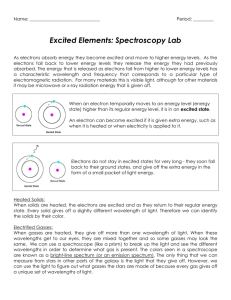
CP Chemistry Laboratory 4 Light, Energy, and Electron Structure Introduction Sunlight passing through a prism produces a rainbow of colors – the visible spectrum. The separation of white light into its component colors occurs when light waves of different wavelengths are bent by different amounts. When a pure atomic gas such as hydrogen or helium is subjected to a high – voltage electrical discharge, light is produced and the gas glows. When this light is passed through a diffraction grating, however, the spectrum it produces is different. Instead of giving the familiar rainbow of colors, the light emitted by the gas gives a series of bright, colored lines. The series of bright lines is called an atomic emission spectrum and is unique to each element. The relationship between the energy of light and its wavelength is E = hc / λ Equation 1 E = _________, h = Plank’s constant = 6.626 *10-34 Js, c = speed of light = _________, λ = _____________ In this experiment you will be using an instrument called a spectroscope to view the line emission spectra for several sources of light. The spectroscope contains a diffraction grating that separates the light into its component wavelengths. Observations will be made of various sources of light with the spectroscope. The emission spectra of elements will be observed from spectrum tubes which contain element in the form of a gas. These gas samples will be exposed to high voltage which will cause the sample to emit the light. This is the same method used in fluorescent lights, street lights, and neon lights. Objective To recognize continuous versus line emission spectra for various sources of light using a spectroscope. To view and record the line emission spectra for several different sources of light. 1 Procedure- You will work with a partner 1. Using the spectroscope observe the continuous “rainbow” spectrum from the incandescent lightbulb. 2. Observe the colors of light in the visible spectrum and the wavelength range for each color band. Sketch the spectrum of white light using colored pencils in the appropriate wavelength boxes in the Spectrum Table. Note that the units of wavelength on the spectroscope are nanometers. (1 nm = 10-9 m) 3. Observe the atomic emission spectrum of air, hydrogen, fluorescent, and two other tubes. In the Data Table make note of the type of spectrum (continuous or line), and the number of lines you observe (only note the bright lines), under the colors column indicate the color and the wavelength of each color. 4. Using the colored pencils sketch the atomic spectrum of the incandescent, hydrogen, and your two other tubes spectra in the wavelength boxes in the Spectrum Table. 5. Answer the post lab questions. 2 Atomic Spectra Report Sheet Data Table Light Source Spectrum Type Colors, Wavelength (nm) (Number of Lines) Fluorescent Light Hydrogen Tube Helium Mercury EXAMPLE: Line Bromine (5 lines) Violet line = 420 nm Violet line = 425 nm Violet = 450 Blue line = 475 nm Blue line = 480 nm 3 Spectrum Table Light Source 700-650 650-600 600-550 550-500 500-450 450-400 nm nm nm nm nm nm Fluorescent Light Hydrogen Tube Helium Tube Mercury Tube Post Lab Calculations and Analysis 1. According to Equation 1 the energy of light emitted (E) is inversely proportional to its wavelength (λ) – as the wavelength increases, its energy decreases (think low, low, long). Based on the spectrum observed for fluorescent white light, rank the colors in the visible spectrum from highest energy to lowest energy. 2. Do all of the colors of light in the visible spectrum span about the same wavelength “width” i.e. do the bands of color appear equally wide or narrow? If not, explain any differences. 3. What color of light in the visible spectrum appears brightest? Does this mean it is the highest energy light? Explain your answer. 4 4. Using Equation 1, calculate the energy (E) corresponding to each line in the atomic emission spectrum of hydrogen. Show your work. 5. ∆ E (J) Color of Line Transition As shown in the figure below, the visible emission spectrum of hydrogen is due to transitions from excited energy levels down to the second principal energy level (n = 2). Thus, the highest energy violet line is due to the transition from n= 6 to n=2, and the lowest energy red line is due to the transition from n=3 to n=2. Enter the energy values (Question 4) from the highest to lowest in the following table and fill in the missing entries. E5 E4 E3 E2 Violet Indigo Blue Red 5 Energy Difference ninitial → nfinal Violet 6→2 Red 3→ 2 6