unit a activity 4c
advertisement

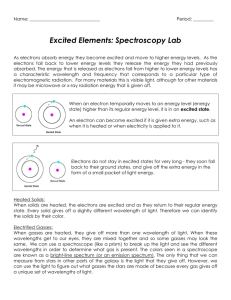
CLASS COPY – Do not remove this paper from the classroom without permission! – CLASS COPY Learning Activity 4c: Spectroscope Lab Unit A: Earth’s Place in the Universe Learning Target: 4c) I can collect and analyze data using a spectroscope (DOK 2) Introduction: How do we know what the Sun or any other star is made of? Many people think that the telescope is the only way to study the stars. But a very important tool to astronomers is the spectroscope. This instrument can tell the astronomer the composition of a star. In this lab you will learn how to collect and analyze data using a spectroscope. Materials: Several light sources (fluorescent light; incandescent light; sunlight*; black light; (other: fiber optic light; plasma ball light; lava lamp) [*reflected – NEVER look directly at the sun for an extended period of time] Spectroscope, rainbow glasses, diffraction grating in a slide mount Handle these materials carefully. Do NOT touch the plastic diffraction gratings in the instruments. Your fingers have oils on them that will fill the small spaces in the plastic, causing blurring of the spectrum you are observing. Colored pencils Procedure: 1. Set up a new entry in your science notebook. Be sure to include a title and a date. Write down a purpose for doing the lab. 2. Listen and look as your teacher demonstrates how to use the different tools in your lab kit. a) Spectroscope – There are 2 ends. The end with the slit gets pointed toward the light source. The end with the diffraction grating – often mounted in a round hole is what you will look through. As you look through the round hole, while pointing the other end (with the slit) at the light source, you should see a band of colors appear INSIDE the tube. You may have to rotate the spectroscope in order to see the spectrum. 3. Try it out. a) First observe the fluorescent lighting that is used in your classroom. Record your observations in your science notebook. Making a table like the one below would be helpful: source Observations (The Spectrum) classroom fluorescent lighting Incandescent light sunlight (reflected) Other light (describe) Element tube (include name of element) Element tube (include name of element) 1 CLASS COPY – Do not remove this paper from the classroom without permission! – CLASS COPY b) Now observe the additional light sources provided by your teacher. You will be in a dark room to minimize stray light from other sources. Record your observations in your science notebook. 4. Look carefully at your data. What patterns do you notice? Record 3-5 statements about your data. 5. Read the information below to better understand what was going on with your spectroscope. Use the information to answer the questions in your science notebook. This is a form of note-taking – be sure to include enough detail so that you can use the information later. A spectroscope works by breaking light into the wavelengths (or spectra) that make it up. Some spectroscopes are made of diffraction grating- a material that has lots of little parallel groves that are approximately one wavelength apart. There may be 35,000 of these little groves in one inch of the material. When light hits the lines, it bends. Different wavelengths (colors) of light bend by different amounts, so it splits the light into its colors. Each color corresponds to a wavelength, measured in nanometers (nm). Note that 1 nm = 10-9m = 0.000000001 m = 1/250 millionth of an inch, a very small unit. The wavelength of visible light is very short indeed. Other spectroscopes are made of prisms- as light passes through the glass, the different wavelengths slow down by different amounts and are bent into their colors. Scientists can tell the elements present in a star by looking at its light through a spectroscope. Each element will have its own unique spectral lines of color, just as people each have a unique fingerprint. Hot, glowing bodies like an incandescent light bulb, or the Sun, glow in all the colors of the spectrum. All these colors together appear as white light. When such white light hits a prism, or a raindrop, or a diffraction grating, colors get separated according to their wavelength. Red, with its wavelength of 600 nm to 700 nm, is deflected least and ends up on one edge of the spectrum – or the rainbow when sunlight hits a raindrop after a storm. Blue, wavelength around 400 nm, is the other end of the visible spectrum. An infinite number of elementary colors are located between these two edges, each corresponding to its own wavelength. This spectrum is called a CONTINUOUS spectrum. However, other physical processes produce different spectra. An element light tube works, crudely speaking, on the principle of lightening. Electrons rush from the negative pole to the positive pole inside, and hit gas atoms in the tube, making them emit light. This sort of light contains only a few colors, and is called an “emission spectrum”. When we separate the colors of such light, only a few bright “emission” lines appear, each in its own color (and wavelength). Each sort of an atom will emit light at its own particular set of wavelengths. When we analyze the emission spectrum of an unknown source, we can compare the colors of its spectral lines to known spectral lines we see in a laboratory, and tell which substance matches. There are 3 types of spectra seen in a spectroscope: continuous all of the colors of the spectrum are present, continuous and blend from one color to another. Like a rainbow. This spectrum is produced from HOT, glowing SOLIDS. emission a series of individual colored lines on a black background. This spectrum is produced from HOT glowing GASES. absorption a continuous spectrum with portions missing. This spectrum is produced when a light source travels through COOL glowing GASES. 2 CLASS COPY – Do not remove this paper from the classroom without permission! – CLASS COPY QUESTIONS: (Remember - Answer the questions in your science notebook. This is a form of note-taking – be sure to include enough detail so that you can use the information later. Include the question in your answer.] 1. Explain – in your own words - what a spectroscope does. 2. Classifying the spectra: Considering the light sources that you observed… a. Which spectrum was a continuous spectrum? How do you know? b. Which spectrum was an emission spectrum? How do you know? c. Which spectrum was an absorption spectrum? How do you know? 3. Making inferences: a. Which light source was burning hot solids? Explain how you determined this. b. Which light source was burning hot gases? Explain how you determined this. c. Which light source was passing through a cool gases? Explain how you determined this. 4. Write (or color) the order of the spectrum from shortest to longest wavelength. longest wavelength shortest wavelength 5. What is a way that you could remember the order of the colors in the continuous visible spectrum? (Ask your teacher about a popular mnemonic device.) 6. A student makes a statement, “The only useful tool astronomers use is a telescope.” Do you agree or disagree with this statement? Explain your thinking. 3