HW#4 - Seattle Central College
advertisement

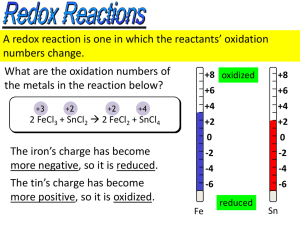
CHEMISTRY 161 HW#4 SOLUTIONS 04, 14, 24, 32, 42, 52, 54, 40, 68, 74, 90, 92, 104, 108. 4-04 (a) Silver in group 11 forms an insoluble halide salt. (b) Hydroxide compounds of both group 11 (such as AgOH) and group 12 [such as Zn(OH) 2] are insoluble. (c) Group 1 elements form compounds with hydroxide (NaOH, KOH, RbOH, CsOH) that are soluble. (d) The binary compounds of group 17 form the compounds HCl, HBr, and HI. These are strong acids. 4-14 0.250 mol 119.00 g = 14.9 g KBr 1L 1 mol 0.200 mol 84.99 g = 0.425 g NaNO3 (b) 0.0250 L 1L 1 mol 0.375 mol 32.04 g = 1.20 g CH3OH (c) 0.1000 L 1L 1 mol (a) 0.5000 L 4-24 1 mol Au 6 109 g Au 196.967 g 2 10 14 M Au 1.5 1021 L 4-32 Placing the values into the dilution equation and solving for Mfinal gives the phosphate concentration: M final Vinitial M initial Vfinal 16 oz 80 g/L 0.313 g/L 128 oz 32 gal 1 gal 4-42 Placing the values into the dilution equation and solving for Mfinal gives the phosphate concentration: M final Vinitial M initial Vfinal 16 oz 80 g/L 0.313 g/L 128 oz 32 gal 1 gal 4-52 (a) HBr(aq) + KOH(aq) H2O ( ) + KBr(aq) Potassium bromide and water are produced. Ionic equation: H+(aq) + Br–(aq) + K+(aq) + OH–(aq) H2O ( ) + K+(aq) + Br–(aq) Net ionic equation: H+(aq) + OH–(aq) H2O ( ) (b) 2 H3PO4(aq) + 3 Ba(OH)2(aq) 6 H2O ( ) + Ba3(PO4)2(s) Barium phosphate and water are produced. Ionic and net ionic equation: 2 H3PO4(aq) + 3 Ba2+(aq) + 6 OH–(aq) 6 H2O ( ) + Ba3(PO4)2(s) (c) Al(OH)3(s) + 3 HCl(aq) AlCl3(aq) + 3 H2O ( ) Aluminum chloride and water are produced. Ionic equation: Al(OH)3(s) + 3 H+(aq) + 3 Cl–(aq) Al3+(aq) + 3 Cl–(aq) + 3 H2O ( ) Net ionic equation: Al(OH)3(s) + 3 H+(aq) Al3+(aq) + 3 H2O ( ) (d) 2 CH3COOH(aq) + Sr(OH)2(aq) 2 H2O ( ) + Sr(CH3COO)2(aq) Water and strontium acetate are produced. 2 CH3COOH(aq) + Sr2+(aq) + 2 OH–(aq) 2 H2O ( ) + Sr2+(aq) + 2 CH3COO–(aq) 2 CH3COOH(aq) + OH–(aq) H2O ( ) + CH3COO–(aq) 4-54 (a) Molecular equation: Al(OH)3(s) + 3 HBr(aq) AlBr3(aq) + 3 H2O ( ) Ionic and net ionic equations: Al(OH)3(s) + 3 H+(aq) + 3 Br–(aq) Al3+(aq) + 3 Br–(aq) + 3 H2O ( ) Al(OH)3(s) + 3 H+(aq) Al3+(aq) + 3 H2O ( ) (b) Molecular equation: H2SO4(aq) + Na2CO3(s) Na2SO4(aq) + H2CO3(aq) Carbonic acid reacts in solution to give CO2(g) + H2O ( ) : H2SO4(aq) + Na2CO3(s) Na2SO4(aq) + H2O ( ) + CO2(g) Ionic and net ionic equations: 2 H+(aq) + SO42–(aq) + Na2CO3(s) 2 Na+(aq) + SO42–(aq) + H2O ( ) + CO2(g) 2 H+(aq) + Na2CO3(s) 2 Na+(aq) + H2O ( ) + CO2(g) (c) Molecular equation: Ca(OH)2(aq) + 2 HNO3(aq) Ca(NO3)2(aq) + 2 H2O ( ) Ionic equation: Ca2+ + 2 OH–(aq) + 2 H+(aq) + 2 NO3–(aq) Ca2+(aq) + 2 NO3–(aq) + 2 H2O ( ) Net ionic equation: OH–(aq) + H+(aq) H2O ( ) 4-40 (a) KCl is 0.025 M K+; 0.025 M Cl– (b) CuSO4 is 0.025 M Cu2+; 0.025 M SO42– (c) CaCl2 is 0.025 M Ca2+; 0.050 M Cl– 4-68 Because a supersaturated solution eventually precipitates the supersaturated solute, honey with a supersaturation of sugar will, upon standing for long periods, precipitate out the sugar, making it cloudy. 4-74 5 10–3 mol mol Cl– 10 mL 5 10–5 mol Cl– 1000 mL Because 1 mol of Cl– reacts with 1 mol of Ag+ to form 1 mol of AgCl, 5 10–5 mol of AgCl forms in the reaction. The mass of AgCl formed is 5 10–5 mol 143.32 g 7 10 –3 g AgCl 1 mol 4-90 A half-reaction describes either just the oxidation reaction (where electrons are lost as a product of the reaction) or just the reduction (where electrons are gained in the reaction and are “reactants” in the reaction). 4-92 Electron gain is associated with reduction half-reactions and electron loss is associated with oxidation half-reactions. 4-104 The oxidation number for sulfur in SO42– and H2S can be determined considering that the typical oxidation numbers for oxygen and hydrogen are –2 and +1, respectively. We can balance the half-reactions for oxidation and reduction using H2O and H+ to balance for oxygen and hydrogen as needed since the reaction is run under acidic conditions. Then, if needed, we multiply by factors to compensate for unequal numbers of electrons and finally add the half-reactions together. 4-108 (a) The half-reactions are MnO4 – MnS S2– S Because we need S for balancing atoms in the first equation, we have to add S 2– to MnO4–. This will preserve the reduction reaction of MnO4– because the S2– we are adding has the same oxidation number as the S atom in MnS. MnO 4 – + S2– MnS S2– S Balancing for O and H (in acidic solution first): 8 H MnO 4 – S2– MnS 4 H 2 O S2– S Balancing for charge: 5 e – 8 H MnO4 – S2– MnS 4 H 2 O S2– S 2 e – Adding the two half-reactions together after multiplying each so the electrons balance: 2 5 e– 8 H+ MnO4 – S2– MnS 4 H 2 O 5 S S 2 e 2– – 16 H (aq) + 2 MnO4 (aq) 2 S (aq) 5 S (aq) 2 MnS( s) 8 H 2 O( ) 5 S( s) + – 2– 2– Adding 16 OH– to both sides to put this into basic solution: 16 OH – 16 H + 2 MnO 4 – 2 S2– 5 S2– 2 MnS 8 H 2O 5 S 16 OH – 16 H 2 O 2 MnO4 – 2 S2– 5 S2– 2 MnS 8 H 2 O 5 S 16 OH – 8 H 2 O( ) 2 MnO4 – (aq) 7 S2– (aq) 2 MnS( s) 5 S( s) 16 OH – ( aq) (b) The half-reactions are MnO 4 – MnO 2 CN – CNO – Balancing for O and H, first in acidic solution: 4 H MnO4 – MnO2 2 H 2 O H 2 O CN – CNO – 2 H Balancing for charge: 3 e – 4 H MnO4 – MnO2 2 H2 O H 2 O CN – CNO – 2 H + 2 e – Adding the half-reactions: 2 3 e – 4 H + MnO4 – MnO2 2 H 2 O 3 (H 2 O CN CNO 2 H 2 e – ) – – + 8 H + (aq) 2 MnO4 – (aq) 3 H 2 O( ) 3 CN – (aq) 2 MnO 2 ( s) 4 H 2 O( ) 3 CNO – (aq) 6 H + (aq ) Simplifying and adding OH– to place the reaction into base: 2 H + 2 MnO 4 – 3 CN – 2 MnO 2 H 2O 3 CNO – 2 OH – + 2 H + 2 MnO 4 – 3 CN – 2 MnO 2 H 2O 3 CNO – 2 OH – 2 H 2 O 2 MnO 4 – 3 CN – 2 MnO 2 H 2O 3 CNO – 2 OH – H 2 O( ) 2 MnO 4 – ( aq) 3 CN – ( aq) 2 MnO 2 ( s) 3 CNO – ( aq) 2 OH – ( aq) (c) The half-reactions are MnO4 – MnO 2 SO32– SO4 2– Balancing for O and H, first in acidic solution: 4 H MnO4 – MnO2 2 H 2 O H 2 O SO32– SO4 2– 2 H + Balancing for charge: 3 e – 4 H + MnO4 – MnO2 2 H 2 O H 2 O SO32– SO4 2– 2 H + 2 e – Adding the half-reactions: 2 3 e – 4 H + MnO4 – MnO2 2 H 2 O 3 (H 2 O SO3 2– SO4 2 H 2 e – ) 2– + 8 H + (aq) 2 MnO4 – (aq) 3 H 2 O( ) 3 SO32– (aq) 2 MnO2 ( s ) 4 H 2 O( ) 3 SO4 2– (aq) 6 H + (aq) Simplifying and adding OH– to place the reaction into base: 2 H + 2 MnO 4 – 3 SO32– 2 MnO 2 H 2 O 3 SO 4 2– 2 OH – 2 H 2 MnO 4 – 3 SO32– 2 MnO 2 H 2 O 3 SO 4 2– 2 OH – 2 H 2 O 2 MnO 4 – 3 SO32– 2 MnO 2 H 2 O 3 SO 4 2– 2 OH – H 2 O( ) 2 MnO 4 – ( aq) 3 SO32– ( aq) 2 MnO 2 ( s) 3 SO 4 2– ( aq) 2 OH – ( aq)