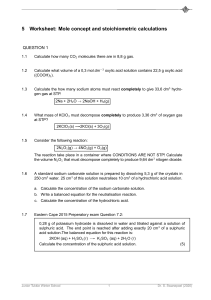
Density is defined as the mass per unit volume
advertisement

Density Density is defined as the mass per unit volume. It is the ratio between the mass and the volume of a substance. It does not matter how large or small a sample of matter is, the same substance will always have the same density, because of this. The ratio between the mass and volume remains the same. This makes density an intensive property. The common units for the density of a solid or liquid are g/mL or g/cm3. A mL and a cm3 are the same. 1 mL = 1 cm3. The common units for the density of a gas are g/L or g/dm3. A L and a dm3 are the same. 1 L = 1 dm3. Therefore, 1L = 1000 mL so 1 dm3 = 1000 cm3. Your textbook uses cm3 and dm3, because these are the SI units of measure for volume. DO NOT let this confuse you. Density One of the ways that problems may be solved is by using an algebraic equation. To solve problems involving density, we use an equation. The equation involves three (3) variables; Density (D), mass (m), and volume (V). If you are given any two of these three variables you should be able to solve for the third variable. Before attempting to solve any density it problems, it may be helpful to solve the density equation for the other variables. Solve the density equation below for the other two variables and then use them to help solve any density problem you may encounter. Density = mass Volume D = m V Solve for m: D•V = m Solve for V: V = m D