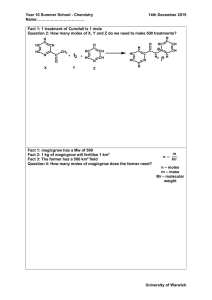
Worksheet Molar Volume of a Gas 1. State Avogadro’s Law. 2. 25 cm3 of hydrogen is burnt in 20 cm3 of oxygen. Write an equation for the reaction. (a)Which of the gases is in excess? By how much? (b)What volume of steam is produced during the reaction? 4. (a) What is the volume occupied at s.t.p. by (i) a mole of nitrogen (ii) 0.25 moles of hydrogen (iii) 0.5 moles of carbon dioxide (iv) 1.5 moles of helium (b) What is the mass of each of the following? (All gases are at s.t.p.) (i) 11.2 dm3 of carbon monoxide? (ii) 5.6 dm3 of oxygen (iii) 22.4 dm3 of chlorine (iv) 112 dm3 of hydrogen (c) What volume would 3 moles of oxygen occupy at room temperature and pressure? 5. Mg + H2SO4 MgSO4 + H2 48g of magnesium reacts with excess dilute sulphuric acid. (a)Why is this acid used in excess? (b)What mass of sulphuric acid is used up? (c)What mass of magnesium sulphate is produced? (d)What volume of hydrogen is liberated?