hmwrk link

Chemistry Measurement Name :
Date :
A. Use chapter 1 as a reference to answer the following questions:
1. The ___________________ system is a system of measurement established in France in 1790.
2. The ___________________ ____________ ( or _____ system) is the system of standards of measurement used by scientists worldwide today.
3. State the basic metric (or SI) units for the following measurements:
_______________ : length
___________ , ________ : volume
_______________ : mass
_______________ : energy (heat)
_______________ : temperature
4. Match the metric prefix with its meaning and symbol
_____ kilo a. one-thousandth(10
-3
)
_____ nano b. one-hundredth(10
-2
)
_____ deci c. one-billionth(10
-9
)
_____ centi d. one-millionth(10
-6
_____ micro e. one-tenth(10
-1
)
_____ milli f. one thousand(10
5. Distinguish between mass and weight.
3
)
) g. k h. c i. n j. d k. u l. m
6. Does an object in space (away from all gravitational forces) have mass or weight or both?
7. ____________________ is the ratio of the mass of an object to its volume.
8. A chunk of copper metal has a mass of 46.76 g and a volume of 5.22 cm
3
. What is its density?
A e
9. What is the mass of a sample of aluminum which has a density of 2.70 g/cm 3 and a volume of 33.0 cm 3 ?
Answer :
10. What is the volume of a sample of lead which has a mass of 44.5 g and a density of
11.4 g/cm 3 ?
Answer :
11. Compare the Kelvin and Celsius temperature scales using the following reference points :
Kelvin Celsius
_______ absolute zero ________
_______ freezing point of water ________
_______ boiling point of water ________
12. Convert the following temperature measurements between the Celsius and Kelvin scales : a. _____ K = 20.0 ºC b. _____ ºC = 40 K c. _____ K = 45.2 ºC d. _____ ºC = 12.0 K k ilo- = 1000 centi- = 1/100 milli- = 1/1000
Conversions
1 kg = 1000 g 1000 mg = 1 g 1 mL = 1 cm 3
1 km = 1000 m 100 cm = 1 m
1 kL = 1000 L
1000 mm = 1 m 1 L = 1 dm
1000 mL = 1 L 1 cm = 10 mm
3
Solve each of the following problems.
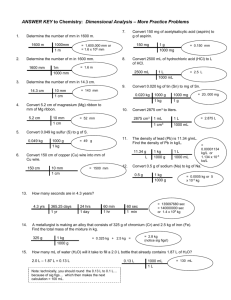
13.
Determine the number of mm in 1600 m.
14.
Determine the number of m in 1600 mm.
15.
Determine the number of mm in 14.3 cm.
16.
How many seconds are in 4.3 years?
17.
Convert 2875 cm 3 to liters.
18.
The density of lead (Pb) is 11.34 g/cm 3 . Find the density of Pb in kg/dm 3 .
19.
Convert 5.2 cm of magnesium (Mg) ribbon to mm of Mg ribbon.
8. Convert 0.049 kg sulfur (S) to g of S.
20.
Convert 0.020 kg of tin (Sn) to mg of Sn.
21.
Convert 150 mg of acetylsalicylic acid (aspirin) to g of aspirin.
22.
Convert 2500 mL of hydrochloric acid (HCl) to L of HCl.
23.
A metallurgist is making an alloy that consists of 325 g of chromium (Cr) and 2.5 kg of iron (Fe). Find the total mass of the mixture in kg.
24.
How many mL of water (H
2
O) will it take to fill a 2 L bottle that already contains 1.87 L of H
2
O?