Methyl Orange Synthesis: Diazo Coupling Lab Manual
advertisement

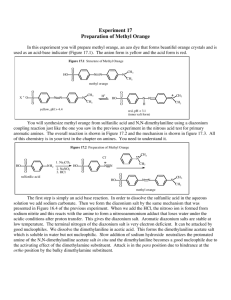
CHEM 334L Organic Chemistry Laboratory Revision 1.0 Diazo Coupling A Synthesis of Methyl Orange In this experiment the azo dye methyl orange is prepared by a electrophilic substitution with arenediazonium salts (diazo coupling). p-Dimethylamino-Azobenzenesulfonic Acid (Methyl Orange) Methyl orange is a pH indicator and due to its clear color change it is very often used in titrations. Methyl orange changes color at the pH of a mid-strength acid and is usually used in titrations for acids. Unlike a so called universal indicator, methyl orange does not have a full spectrum of color change, but has a sharper end point. Space-filling model of methyl orange Powder of methyl orange Left: Methyl orange in an acidic solution Right: Methyl orange in an basic solution http://en.wikipedia.org/wiki/File: Methyl-orange-3D-vdW.png http://en.wikipedia.org/wiki/File: Methyl-orange-sample.jpg http://en.wikipedia.org/wiki/File: Methyl_orange_02035.JPG Methyl orange is prepared from sulfanilic acid and N,N-dimethylaniline. The first product obtained from the coupling is the bright red acid form of methyl orange, called helianthin. In base, helanthin is converted to the orange sodium salt, called methyl orange. P age |2 Dyes are used to give colors to substances, especially fabrics. Chromopohores, functional groups that absorb light, give color to these dyes. The most common chromophores are azo, nitro, and carbonyl groups. Auxochromes, functional groups that increase the intensity of the color, are also important parts of dyes. The most common chromophores are hydroxyl, amino, sulfonate, and carboxylate groups. Azo dyes have a nitrogen to nitrogen double bond as their chromophore. These dyes are created by taking a diazonium salt and adding it to a strongly activated aromatic system. In this experiment, you will synthesize methyl orange, an azo dye, by a diazonium coupling reaction with diazotized sulfanilic acid and N,N-dimethylaniline. Preparation of the diazonium salt of sulfanilic acid Although sulfanilic acid is insoluble in acid solutions, it is nevertheless necessary to carry out the diazotization reaction in an acid (HNO2, nitrous acid) solution. This problem can be circumvented by precipitating sulfanilic acid from a solution in which it is initially soluble. The precipitate which is formed is a fine suspension and reacts instantly with nitrous acid. The first step is to dissolve sulfanilic acid in basic solution. Formation of nitrosonium ion In order to obtain the nitrosonium ion (NO+), sodium nitrite has to be treated with hydrochlorid acid. During the addition of the acid, the sulfanilic acid is precipitated out of solution as a finely divided solid, which is immediately diazotized (see below). P age |3 Formation of diazotized sulfanilic salt Addition to N,N-dimethylaniline The finely divided diazonium salt is allowed to react immediately with dimethylaniline in the solution in which it was precipitated. P age |4 Methyl orange is often used as an acid-base indicator. In solutions that are more basic than pH 4.4, methyl orange exists almost entirely as the yellow negative ion. In solutions that are more acidic then pH 3.2, it is protonated to form a red dipolar ion. Due to these properties, methyl orange can be used as an indicator for titrations that have their end points in the pH 3.2 to 4.4 region. The indicator is usually prepared as a 0.01% solution in water. In higher concentrations in basic solution, of course, methyl orange appears orange. P age |5 Pre-Lab Questions 1. Look up the structure of Alizarin Yellow. How might this Azo Dye be prepared in a Diazo coupling reaction? Alizarin Yellow is a pH indicator. What are the colors of the acidic and basic form of this indicator? 2. Why does the dimethylaniline couple with the diazonium salt at the para position of the ring? 3. The diazonium coupling reaction is an electrophilic aromatic substitution reaction. Give a mechanism that clearly indicated this fact. Show all the important resonance contributors. P age |6 Procedure Diazotized Sulfanilic Acid 1. Dissolve 1.1 g of anhydrous sodium carbonate in 50 mL of water. Use a 125-mL Erlenmeyer flask. 2. Add 4.0 g of sulfanilic acid (3.6 g if anhydrous) monohydrate to the solution and heat it until it dissolves. A small amount of suspended material may render the solution cloudy. 3. Gravity-filter the still hot solution and rinse the used filter paper with a little (2-5 mL) hot water. 4. Cool the filtrate to room temperature, add 1.5 g of sodium nitrite, and stir until solution is complete. 5. Pour this mixture, while stirring, into a 400-mL beaker containing 25 mL of ice water to which 5 mL of concentrated hydrochloric acid (HClconc.) have been added. The diazonium salt of sulfanilic acid should soon separate as a finely divided white precipitate. Keep this suspension cooled in an ice bath until it is to be used. Methyl Orange 6. In a test tube, mix together 2.7 mL of dimethylaniline and 2.0 mL of glacial acetic acid. 7. Add this solution to the cooled suspension of diazotized sulfanilic acid in the 400mL beaker. Stir the mixture vigorously. In a few minutes, a red precipitate of helianthin should form. Keep the mixture cooled in an ice bath for about 15 minutes to ensure completion of the coupling reaction. 8. Add 30 mL of a 10% aqueous sodium hydroxide (NaOH) solution. Do this slowly and with stirring, as you continue to cool the beaker in an ice bath. Check with litmus or pH paper to make sure the solution is basic. If it is not, add more base. 9. Heat the mixture to boiling with a Bunsen burner for 10 to 15 minutes to dissolve most of the newly formed methyl orange. When all (or most of it) the dye is dissolved, add 10 g of sodium chloride, and cool the mixture in an ice bath. The methyl orange should recrystallize. P age |7 10. To purify the product, transfer the filter cake and paper to a large beaker containing about 150 mL of boiling water. Maintain the solution at a gentle boil for a few minutes, stirring it constantly. Note: Not all the dye will dissolve, but the salts with which it is contaminated will dissolve. 11. Remove the filter paper and allow the solution to cool to room temperature. Cool the mixture in an ice bath, and when it is cold, collect the product by vacuum filtration, using a Buchner funnel. Allow the product to dry, weight it, and calculate the percentage yield. Note: The product is a salt. Since salts do not generally have well-defined melting points, the melting-point determination should not be attempted. Spectroscopy 1. Obtain a NMR spectrum of the product. Consult with your laboratory instructor about how to do this. 2. Assign all the NMR peaks in the spectrum. P age |8 Post-Lab Questions 1. Give the products of diazo coupling of benzenediazonium chloride with each of the following molecules: (a) Methoxybenzene (b) 1-Chloro-3-methoxybenzene (c) 1-(dimethylamino)-4-(1,1-dimethylethyl)benzene Hint: Diazo couplings are quite sensitive to steric effects. 2. Look up the structure of (a) Prontosil and (b) Congo Red. Show the reagents that would be necessary for the synthesis by diazo coupling of each of the two compounds.