Uploaded by
Meow Cads
Methyl Orange Synthesis Lab Report

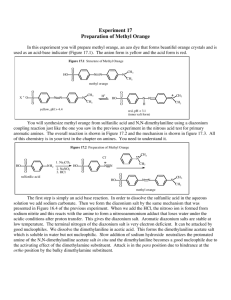
Experiment 1: SYNTHESIS OF METHYL ORANGE REACTION MECHANISM Merrene Bright D. Judan (BSIP, 2015-00957) Methyl orange is prepared from sulfanilic acid and N,N-dimethylaniline. The first product obtained from the coupling is the bright red acid form of methyl orange, called helianthin. In base, helanthin is converted to the orange sodium salt, called methyl orange. Azo dyes have a nitrogen to nitrogen double bond as their chromophore. These dyes are created by taking a diazonium salt and adding it to a strongly activated aromatic system. Although sulfanilic acid is insoluble in acid solutions, it is nevertheless necessary to carry out the diazotization reaction in an acid (HNO2, nitrous acid) solution. This problem can be circumvented by precipitating sulfanilic acid from a solution in which it is initially soluble. The precipitate which is formed is a fine suspension and reacts instantly with nitrous acid. 1. Preparation of diazonium salt of sulfanilic acid: Dissolve sulfanilic acid in basic solution. 2. Formation of nitrosonium ion: In order to obtain the nitrosonium ion (NO+ ), sodium nitrite has to be treated with HCl. 3. Formation of diazotized sulfanilic salt: nitrosonium ion + diazonium salt 4. Addition to N,N-dimethylaniline: The finely divided diazonium salt is allowed to react immediately with dimethylaniline in the solution in which it was precipitated. Net reaction: In summary: Reference: Altig, J. (n.d.). Diazo coupling: Synthesis of methyl orange. In Chem 334L: Organic chemistry laboratory. Retrieved from http://infohost.nmt.edu/~jaltig/Diazo.pdf Long Island University (n.d.). Experiment 17: Preparation of methyl orange. Retrieved from http://myweb.brooklyn.liu.edu/swatson/Site/Laboratory_Manuals_files/Exp17.pdf