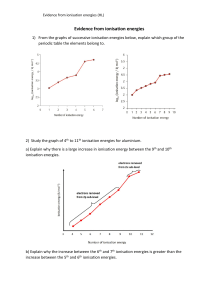
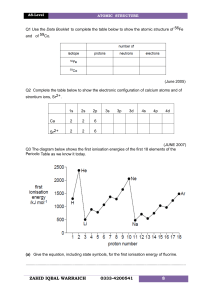
Chemistry: Holiday HW Class: 11AS Date: 16 /04/2023 1. 2. Why is the first ionisation energy of phosphorus greater than the first ionisation energy of silicon? A. A phosphorus atom has one more proton in its nucleus. B. The atomic radius of a phosphorus atom is greater. C. The outer electron in a phosphorus atom is more shielded. D. The outer electron in a phosphorus atom is paired. 3. 4. 5. 1 6. 7. 8. 9. 2 10. (a) Construct an equation to represent the first ionisation energy of oxygen. include state symbols. .................................................................................................................................... [1] (b) (i) State and explain the general trend in first ionisation energies across Period 2. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. [3] (ii) Explain why ionisation energy A in Fig. 1.1 does not follow the general trend in first ionisation energies across Period 2. ................................................................................................................................................... ................................................................................................................................................... .................................................................................................................................................... ..................................................................................................................................................... [2] 3 11. 4 5 12. 13. 6 14. 7 8 9