Chapter 2
advertisement

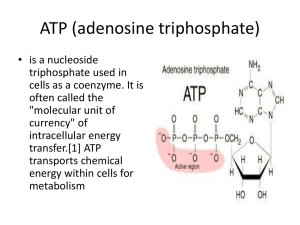
Lecture: Basic Chemistry I. Matter and Energy A. Matter - fundamental building blocks of nature 1. elements - basic units of matter B. Energy - capacity to do work (put matter into motion 1. potential energy - energy stored in a structure a. water stored in a lake uphill b. chemical bonds of glucose molecule 2. kinetic energy - energy in an object in motion a. water in a stream - allows mill to grind corn b. broken glucose bonds -> ATP -> muscles work 3. Forms of Energy a. chemical energy - energy in chemical bonds I. ATP (adenosine triphosphate) - stores energy b. electrical energy - energy of separated charges I. battery - + pole and - pole separate charge ii. nervous impulse run just like a battery c. mechanical energy - energy of matter in motion I. bowling ball transfers energy to move pins ii. muscle motion - ATP -> contraction of muscle d. electromagnetic energy - energy traveling in waves (light, X-rays, UV rays) I. electromagnetic spectrum - visible light, UV light, radio waves, X-rays C. First Law of Thermodynamics 1. "Energy can change from one form to another, but it can never be created or destroyed" (Total Energy In = Total Energy Out) examples: Car Engine vs. Human Body a. Car Engine - gasoline used to run motor to move car Chemical Energy (gas) ---> motion (20%) + heat (79%) + sound (1%) b. Human Body - food used to move body, digest, think, etc. Chemical Energy (food/glucose) ---> physiology (80%) + heat (20%) II. Organization of Matter (Atoms - Elements) A. Atomic Particles Mass Charge Characteristics proton 1 +1 defines element neutron 1 neutral defines isotopes electron 0 -1 determines element bonding properties B. Organization of Periodic Table 1. # protons = atomic number (unique for each element) 2. # protons + # neutrons = atomic mass 3. isotope - same element; different # neutrons # protons Carbon-12 (99%) Carbon-13 (0.9%) Carbon-14 (0.1%) 4. + # neutrons = 6 6 6 6 7 8 atomic mass 12 13 14 # electrons - dictates the NET CHARGE of an atom H H+ H- # protons # electrons 1 1 1 1 0 2 NET CHARGE 0 +1 -1 ion - any atom with a positive or negative charge anion - atom with a NEGATIVE charge cation - atom with a POSITIVE charge III. Electron Shells, the Periodic Table, and Chemical Bonds A. Electron Shells - electrons occupy "shells" as they orbit around the nucleus (2, 8, 8, ...) B. The Periodic Table of Elements is organized by electron shells H1 Li3 Be4 Na11 Mg12 He2 B5 C6 N7 O8 F9 Ne10 SHELL 2 Al13 Si14 P15 S16 Cl17 Ar18 SHELL 3 SHELL 1 8 e8 e- 2 e- C. Chemical Bonds are formed so that each atom can have the outermost electron shell filled 1. Ionic Bond - one atom donates electron(s) to another a. Example: Sodium Chloride (table salt) Na+Cl- 2. Covalent Bond - two atoms share one/more electrons a. Example: Methane (CH4), Carbon Dioxide (CO2), and Ammonia (NH3) a. Polar Molecule - electron sharing is unequal in the bonds Example: Water (H2O) b. Non-polar Molecule - electron sharing is almost equal Example: Methane (C02) IV. Elements other than C,H,O,N in Humans Primary Elements (3% of all body weight) Calcium Ca Bones,teeth, muscle and nerve action, blood clotting Phosphorus P Bones and Teeth, DNA, RNA, ATP. Important in energy transfer Trace Elements (Less than 1% of body weight altogether) Potassium K Osmotic balance; cell voltage, muscle and nerve action Sulfur S Component of proteins (cysteine) and other organic molecules Sodium Na Osmotic balance; cell voltage, muscle and nerve action Chlorine Cl Osmotic balance; cell voltage, muscle and nerve action Magnesium Mg Co-factor for many enzymes Iron Fe Hemoglogin and many enzymes Copper Cu Co-factor of many enzymes Zinc Zn Co-factor of many enzymes Manganese Mn Co-factor of many enzymes Cobalt Co Co-factor of many enzymes and vitamin B12 Chromium Cr Co-factor of many enzymes and potentiates Insulin Selenium Se Required for normal liver function Molybdenum Mo Co-factor of many enzymes Flourine F Tooth and bones Tin Sn Promotes growth (unknown mechanism) Silicon Si Growth, bone mineralization, connective tissue synthesis Vanadium V Promotes growth and reproduction V. Chemical Reactions A. Patterns of Chemical Reactions 1. Chemical Equation - # of atoms of each element same for reactants and products C6H12O6 + 6O2 ----------> 6H2O + 6CO2 2. Synthesis - smaller molecules form larger molecule A + B ----> AB (anabolic process) amino acid 1 + amino acid 2 + ........ ----> peptide (protein) sugar 1 + sugar 2 + sugar 3 + .......... -----> polysaccharide (glycogen) 3. Decomposition - larger molecule broken down into smaller molecules AB ----> A + B glycogen ----> glucose + glucose + glucose + .......... 4. Displacement - one part is exchanged AB + C -----> A + BC glucose + adenosine-P-P-P (ATP) -------> glucose-P + adenosine-P-P (ADP) B. Exergonic vs. Endergonic Reactions 1. Exergonic - energy is released during the reaction A + B ------> C + D + ENERGY glucose + oxygen ----> water + carbon dioxide +ENERGY (trapped by ATP) 2. Endergonic - energy required for reaction to proceed A + B + ENERGY ------> C amino acid 1 + amino acid 2 + ... + ENERGY ---> peptide (protein) C. Chemical Equilibrium 1. Reversible Reactions A + B ---------> AB and AB --------> A + B 2. Chemical Equilibrium A + B <=====> AB D. Rates of Chemical Reactions 1. size of reactants species (smaller means faster) 2. temperature (speeds up the particles) 3. concentration (more likely to come together) 4. catalysts (enzymes) - make reacting more convenient VI. Acid- Base Chemistry and the pH Scale A. Water normally exists in an equilibrium reaction with some dissociation H2O <======> H+ + - OH in a beaker of pure water, the ratio of H+ to H2O is about 1/10,000,000 pH = -log10[H+] = -log10[10-7] = -(-7) = 7 pH = relative concentration of H+ in a solution of water B. Acids - compounds which increase the concentration of H+ (pH = 1 to 6) C. Bases - compounds which decrease the concentration of H+ (pH = 8 to 14) D. Buffer - compound that prevents large changes in pH of a solution (pH “shock absorber”)