Ch 7 Spectroscopy 08

Year 12 Chemistry Chapter 7: Spectroscopy
7.1 The Electromagnet Spectrum
All forms of spectroscopy use a part of the electromagnet spectrum to give us information about the materials around us. Electromagnet radiation interacts with atoms and molecules.
The various spectroscopic techniques provide us with information about:
The type of atom or molecule that is present ( _________________ analysis)
How much of a particular atom or molecule is present ( _______________ analysis)
The structure and bonding of the molecule.
Spectroscopic techniques utlise the fact that:
Atoms and molecules absorb and emit electromagnetic radiation of specific energies.
Atoms and molecules undergo change when they absorb electromagnetic radiation.
Different parts of the electromagnetic spectrum affect different parts of the atom or molecule.
Radiation from each portion of the electromagnet spectrum has a specific frequency and wavelength and energy associated with it.
The atom or molecule absorbs a specific quantum of energy (packet), which causes the atom or molecule to move to a higher energy level. With atoms, we look at the movement of electrons to higher energy levels. With molecules, as well as the movement of
electrons to higher energy levels, we observe the movement of molecules to higher
vibrational, rotational and nuclear energy levels.
Spectroscopic techniques
Ultraviolet spectroscopy (UV)
Colorimetry
Part of the electromagnetic spectrum
Wavelenght range (cm)
Part of atom or molecule affected
Atomic Absorption (AAS) Spect
Atomic Emission (AES) Spect
Flame test
Infrared Spectroscopy (IR)
Nuclear magnetic resonance spectroscopy (NMR)
7.2 Analysis of Atoms
Flame tests, atomic emission spectroscopy (AES) and atomic absorption spectroscopy
(AAS) use radiation from the visible region of the electromagnetic spectrum.
Flame test
Chemists use the fact that some metals produce particular colours when they are heated as a convenient method of analysis. The metal is identified by comparing the flame colour with a known list of the characteristic colours produced by metals.
To understand how the colour is produced you must look at the structure of the atom. In this method a central nucleus is surrounded by electrons moving in regions called shells.
Shells are also called _______ _______, and electrons in these shells, are located at different distances from the nucleus.
Electons in energy levels close to the nucleus have the lowest energies and experience the strongest attraction to the nucleus. An electron can jump to a higher energy level if it absorbs energy that corresponds exactly to the difference in energy between the lower energy level and the higher energy level.
Higher energy levels are unstable so the ____________ electrons quickly return to lower energy levels (ground state – original energy level) emitting the energy they had absorbed being seen as coloured light.
Electrons in an atom can be excited to a number of higher energy levels, so light emitted from a sample placed in a flame is likely to by a mixture of several different colours
(and energies).
Atomic Emission Spectroscopy (AES)
Two modifications:
Uses a hotter flame, so that sufficient energy is available to excite electrons in a wider range of elements.
Light is passed through a prism. The different energies in the light emitted by a heated sample are separated into a series of coloured lines.
*Figure 7.8
Because each element has a different number of protons in the nucleus, the attraction of the nucleus for electrons will vary from element to element. No two elements will therefore have energy levels of exactly the same energy so a spectrum is characteristic of a particular element.
Each line in the emission spectrum corresponds to radiation of a specific wavelength, frequency and energy exactly equal to the difference in energy of the electron energy levels.
The use of atomic emission spectroscopy, allows for a more certain qualitative analysis.
7.3 Atomic Absorption Spectroscopy
In the early 1950’s, the Australian scientist Alan Walsh came up with the idea of looking at the light absorbed by atoms, which is much more sensitive and accurate than atomic emission spectroscopy, and that can be used to identify and measure a much wider range of metals than AES. This new method is called atomic absorption spectroscopy (AAS).
Very versatile, being able to detect over 70 elements
Extremely sensitive, detecting concentrations of elements at parts per million
(ppm) levels, or in some cases, parts per billion (ppb).
There are many uses of AAS:
Analysis of magnesium in blood
Mercury in fish (maximum limit 0.5 ppm)
Urine and blood analysis to detect excess or deficiency of metals
Detection of toxic metals in blood
Environmental sampling
Assays of mineral and soil samples
Analysis of metals present in engine oil.
How atomic absorption spectroscopy works
Atoms will absorb light if the energy of the light is exactly that required to promote an electron from its ground state (normal energy level) to a higher energy level. Each element analysed requires its own light source that will emit light of the correct wavelength. The light is provided by a special lamp called a hollow source cathode lamp.
The cathode is composed of the element of interest.
A solution of the ample to by analysed is sprayed into ta flame where it is converted into an atomic vapour.
Light containing the chosen wavelength is passed through the flame
Atoms of the element being analysed that are present in the flame absorb some of the radiation.
The light beam is then passed through a filter (monochromator) to select light of the chosen wavelength and its intensity is measure by a electronic detector
The amount of light absorbed indicates the quantity of the element present in the original sample.
Interpreting atomic absorption spectra
A batch of oysters was suspected of containing dangerous levels of lead.
One of the oysters was removed from its shell and chopped finely. A 1.50 g portion was heated with 10 ml of nitric acid. This mixture was filtered and then sprayed into an atomic absorption spectrometer. A significant amount of light was absorbed, indicating that there was lead in the oyster.
To find the concentration of lead ions in the sample, the absorbance of different solutions containing known concentrations of lead ions was also measured. The results are graphed below (calibration curve).
The recommend limit of lead in oysters for human consumption is 2.5 ppm. Are these oyster fit to eat?
Solution: Looking at the graph, the concentration of lead in the solution prepared from the oyster sample is _____ ppm, which is equivalent to 9.0 g/ml.
So in the 10 mol solution there is 9.0 x 10ml = 90 g.
Since there was 90 g of lead present in the 1.50 g portion of oyster:
Concentration in the oyster = 90 g/ 1.50 g
= 60 g/g or 60 ppm
OSYTERS ARE NOT FIT FOR HUMAN CONSUMPTION!
In a solid sample:
2.5 ppm = 2.5
g per g of sample
= 2.5 mg per kg of sample
In an aqueous sample:
2.5 ppm = 2.5
g per ml of sample
= 2.5 mg per L of sample
Year 12 Chemistry: Chapter 7:~ UV-vis and IR Spectroscopy
7.4 UV-visible Spectroscopy
A UV-visible spectrophotometer makes use of the fact that many substances absorb light of characteristic wavelengths. The wavelengths of the light absorbed by compounds can be used for their identification.
UV-visible spectra arise because photons in this region of the electromagnetic spectrum have sufficient energy to promote electrons from low energy levels to higher energy levels. Such electron transitions may occur in atoms, ions or molecules.
When a substance absorbs visible light, it appears coloured. The colour observed is the
complement of the absorbed colour because this is what remains and reaches our eyes.
For example: chlorophyll appears green because its molecules absorb violet and red light.
UV-visible spectroscopy is mainly used for determining the concentration of a substance in a sample. This procedure involves recording the spectrum of the pure substance and selecting a wavelength at which the substance absorbs strongly but other components in the sample do not.
UV-visible spectroscopy is used in:
Hospital pathology labs use UV-vis to determine the haemoglobin content and sugar levels in blood.
Determining the amount of coloured dye of plastics
Identifying the presence of metal ions
Determining the levels of nutrients, additives and contaminants in water and foods
In quantitative analysis of DNA and proteins in the field of molecular biology.
The radiation source provides ultraviolet and visible light of all wavelengths.
The monochromator selects a particular wavelength from those emitted by the source, and the detector measures the intensity of the light that passes through the sample.
Usually the substance under investigation is in solution. It is placed in the spectrophotometer in a special cell made of quartz or fused silica transparent to ultraviolet and visible light. A reference reading is first taken with a cell containing only pure solvent. The reference cell is then replaced with a cell containing a solution of the sample. The absorbance by the sample is found by comparing the two readings. By measuring the absorbance at various wavelengths a graph or spectrum for the sample can be obtained.
There is a more sophisticated machine that chops the beam rapidly between the reference cell and the sample cell: double beam scanning spectrophotometer.
*Check out Figure 7.17
Interpreting ultraviolet-visible spectra
In ultraviolet-visible spectroscopy, the absorbance of visible or ultraviolet light by series of standard solutions is measured and a calibration graph is drawn.
Example: The concentration of iron in a sample of dam water was determined by UV spectroscopy. Iron presents as Fe 2+ ions was reacted to form an orange-yellow complex, iron (II)-1, 10-phenanthroline. The absorbance of a series of standards and a sample of dam water is shown below. Determine the concentration of iron in the dam water in
g/ml (ppm).
The first step is to construct a calibration graph with the above data. The concentration of iron is plotted on the horizontal axis and the absorbance on the vertical axis.
The dam water was not diluted before being treated and analysed so the concentration of the iron in the dam water was _______ g/ml or ____ ppm Fe 2+ .
Questions: 3, 4, 5, 23, 24, 9, 10, 11, 12, 26, 36 & 38.
7.5 Infrared Spectroscopy (IR)
Infrared radiation is lower in energy and of a longer wavelength than visible and ultraviolet light. The energy from infrared radiation is not enough to promote electrons to a higher energy level, but it is enough to cause changes to the bonds in molecules.
Covalent bonds can be compared to springs that undergo specific amounts of bending or stretching. Molecules are only able to occupy discrete vibrational energy levels. A molecule will absorb a discrete quantum of energy to move from one vibrational energy level to the next. The amount of energy required is provided by radiation in the infrared region of the electromagnetic spectrum.
Infrared spectroscopy is a powerful analytical technique because, apart for a few homonuclear molecules such as O
2
and N
2
, all molecules absorb infrared radiation. For a molecule to absorb infrared radiation the bending or stretching vibration must change the overall dipole moment of the molecule.
Generally, the range of energies absorbed depends on the strength of the bonds. A single C-C bond absorbs lower-energy infrared radiation than a triple C C bond.
The mass of the atoms attached by the bond also affects the energy of the infrared radiation absorbed: the higher the mass, the lower the energy of radiation absorbed.
These characteristics of the infrared spectrum make it a powerful tool for identifying the bonds of molecules.
Instrumentation
Features of a dispersive infrared spectrophotometer are:
A source of infrared radiation
Sample and reference cell or disc made out of NaCl, KBr or similar (glass and plastic cannot be used as they absorb IR radiation.
A wavelength selector
An infrared detector
The difference in transmittance or transmitted radiation, between the sample and reference cell is due to the absorption of certain frequencies by the molecules of the sample. These absorptions result in changes in vibrational energy in the molecule under examination.
Interpreting the infrared spectrum
Qualitative analysis
Infrared spectroscopy is most important for the information it can give us about the
functional group.
Functional groups are the atoms or groups of atoms that determine the properties and reactions of an organic molecule
.
The energy of radiation absorbed and released depends on:
The kind of bonds in the molecule. Each type of bond absorbs infrared radiation over a typical narrow range of wavelengths. Example: it takes a different amount of energy and so a different range of wavelengths of radiation to stretch a C-H bond compared to a C-C bond.
Gaseous Ethanol
Bond vibrations in ethanol and its infrared spectrum.
The environment or other bonds in the molecule. The wavelength absorbed depends upon the entire molecular structure. Propanoic acid and methyl ethanoate have the same molecular formula (C
3
H
6
O
2
) but different structures. Both spectra have an absorption band at about 1700 cm -1 due to the C O bond. In methyl ethanoate the absorption just below 3000 cm -1 is due to the C-H bond. In propanoic acid the broad absorption band from 2700 cm-1 to 3600 cm-1 due to the O-H bond. This broad absorption band partly masks the absorption due to the
C-H bonds. The C O stretch in propanoic acid produces a peak at a different wavenumber from the C O stretch in methyl ethanoate. a b
No two molecular compounds are identical in bonds and bonding environment, and so IR spectroscopy can give us a characteristic fingerprint for each compound.
Some of the peaks in a IR spectrum are narrow. This usually means that the peak corresponds to one particular type of vibration of a bond. Other peaks are broad.
These broad bands are combinations of peaks due to a number of vibrational changes
that have overlapping energies. The IR spectrum above 1000 cm
functional groups. The spectrum at frequencies less than 1000 cm
-1
-1
is used to identify
is characteristic of a particular compound.
Also in your data booklet.
*The table above indicates the wavenumber regions where a bond absorbs radiation.
Note that the precise wavenumber of the absorption depends upon the molecular structure of the compound.
Example: The structural formula and IR spectrum of ethanoic acid are shown below. The bond types present in ethanoic acid can be identified from the structural formula.
Peak A is a broad peak with a maximum absorption at 3100 cm -1 = ________________.
The peak at 2990 cm -1 produced by C-H bond is partly masked by the O-H peak and is of limited use. Peak c at 1750 cm -1 is indicative of ______ bond.
The set peaks below 1000 cm -1 is more difficult to interpret. This set is unique to ethanoic acid and is known as the fingerprint region.
Quantitative analysis
If one peak in the spectrum is selected, the absorbance increases as the concentration of the molecule in the sample increases. This means IR spectroscopy can be used for quantitative analysis. A calibration curve is constructed by using standards of known concentration. The concentration of the sample is found by comparison with the calibration curve. Unfortunately calibrations are often significantly curved rather than linear. Quantitative analysis of toxic gases in the atmosphere such as sulfur dioxide, hydrogen cyanide and carbon disulfide is often carried out by IR spectroscopy.
READ: pg 95 ‘IR spectroscopy and breath tests’
Questions: 14, 15, 28, 29 & 39.
Year 12 Chemistry: Chapter 7:~ Nuclear Magnetic Resonance Spectroscopy
7.6 Nuclear Magnetic Resonance Spectroscopy (NMR)
Nuclear magnetic resonance spectroscopy (NMR) is one of the most powerful techniques for determining the structure of complex biochemical molecules. NMR uses energy in the radio frequency range of the electromagnetic spectrum. The energy of the radiation is too low to cause electronic, vibration or rotational transitions. The radio waves used in NMR cause a change in the ‘spin’ of particles in the nucleus. Protons, neutrons and electrons can be thought of as spinning on their axes in either an ‘up’ ( ) or
‘down’ ( ) direction. Nucleons (protons and neutrons) must have an odd number of nucleons, such as 1 H or 13 C, the nucleus always has an overall spin.
A spinning charged particle creates a tiny magnetic field along its axis. When an external magnetic field is applied, the tiny magnetic field of a spinning nucleon aligns either with the field (lower energy) or against the field (higher energy).
A nucleon can absorb a quantum of energy in the radio region and be promoted to the higher energy spin energy level. When it relaces back to the lower energy level, the same radio frequency is emitted.
The most common form of NMR is based on the hydrogen-1 ( 1 H), nucleus, or proton, and is called proton NMR. It can give information about the structure of any molecule containing hydrogen atoms. NMR uses the 13 C isotope often due to the large number of complex biochemicals containing carbon.
The difference in the two spin states depends on:
The identity of the nucleus, eg 1 H is different from
The chemical environment surrounding the nucleus.
13 C,
The amount of nuclear shielding depends on the other atoms surrounding the nucleus.
This in turn affects the amount of energy needed for the nucleus to change its spin. For example, hydrogen in –CH
2
- will absorb at a slightly different frequency from hydrogen in –CH
3
- or hydrogen in –OH-.
To standardise measurements on different instruments and under different conditions, a reference sample , usually tetramethylsilane (TMS), (CH
3
)
4
Si, is used. TMS has a symmetrical tetrahedral structure and therefore all the atoms in the molecule are in an identical environmental. A single peak is formed.
The difference in energy needed to change spin state in the sample is compared to TMS and is called the chemical shift, and is measure in ppm.
Instrumentation
A radio transmitter coil that produces a short powerful pulse of radio waves.
A powerful magnet that produces strong magnet fields
The sample is placed in a glass tube that spins so the test material is subject to a uniform magnetic field. Solid samples are dissolved in a solvent (that will not give a signal, therefore do not have a magnetic dipole moment).
spectrum.
A radio receiver soil that detects the radio frequencies emitted as nuclei relax to a lower energy level.
A computer that analyses and records the data
Proton NMR
Interpreting the NMR spectrum (low and high resolution spectra)
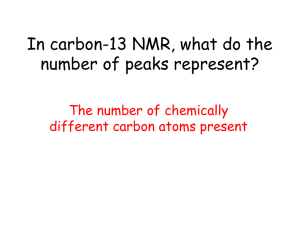
Proton NMR is used to identify the number of chemically distinct hydrogen
‘environments’ there are in a molecule. In low resolution proton NMR the number of
peaks is equal to the number of different bonding environments experienced by the
hydrogen nuclei in the molecule.
In propanone, CH3COCH3, all six hydrogen atoms are in –CH3, and both CH3 are bonded
to the C O. They are all in the same environment so there is only one peak in the NMR
In dimethoxymethane, CH
3
OCH
2
OCH
3
, a total of six hydrogen atoms is in the two
–CH
3
groups and both are attached to the O. Two hydrogen atoms are in a –CH
2
- group.
There are two peaks in the NMR spectrum as there are two different environments for the hydrogen atoms. The area under the –CH
3
peak is three times bigger because there are six hydrogen atoms in that environment compared with the two hydrogens in the
–CH
2
- environment.
In ethanol, CH
3
CH
2
OH, there are three hydrogen atoms in CH
3
, two hydrogen atoms in
CH
2
and one hydrogen in OH. The low resolution NMR spectrum thus has three peaks.
The area under each peak is proportional to the number of hydrogen atoms in each environment.
At high resolution the NMR spectrum of ethanol shows more detail. The peak due to
–CH
3
protons is split into three fine peaks, while the peak due to –CH
2
- protons is split into four fine peaks.
The number of peaks caused by splitting equals n + 1, where n is the number of H atoms on the neighbouring atom, ie:
CH splits the signal form hydrogens attached to adjacent atoms into two peaks.
CH
2
splits the signal from hydrogens attached to adjacent atoms into three peaks.
CH
3
splits the signal from hydrogens attached to adjacent atoms into four peaks.
Summarising:
Chemical shift provides information about the functional group to which the hydrogen atom is attached
Peak splitting gives information about the neighbouring protons.
Peak areas give information about the number of equivalent hydrogen atoms.
*READ: pg 100 ‘Magnetic resonance imaging’
Interpreting of NMR spectra
The proton NMR spectrum of a compound, molecular formula C
4
H
8
, is shown below:
Try to draw the structure:
Peak set A
Relative area Peak split Chemical shift
Peak set B
There are two peaks, therefore _____ different types of H environments
Peak A: has _____ fine peaks so the adjacent carbon, peak set B, must have
_____ hydrogens in the group. Peak set B is therefore due to _____ .
Peak set B is split into _____, so the adjacent carbon, peak set A, must have
____ hydrogen in the group. Peak set A is therefore due to _____
For every one hydrogen in peak set A there are three hydrogens in peak set B, so for every CH group there is a CH
3
group.
Refer to Table 7.9 or data booklet to use the chemical shift to identify the group.
Chemical shift for peak set B is _____, and is consistent for CH3 attached to a
_____ group.
Chemical shift for peak set A is _____ and is consistent with CH attached to a
____ group by a double bond.
The likely structure for the compound is CH3CH CHCH3, but-2-ene.
*Draw the structure:
13C NMR Spectroscopy
Carbon-13 is a naturally occurring isotope of carbon that has nuclear spin. It is used in
NMR spectroscopy to identify different carbon atom environments within a molecule.
The chemical shift from the TMS reference is dependent on a carbon atom’s environment within a molecule. The peaks in the spectrum are a single line produced for each different carbon atom environment.
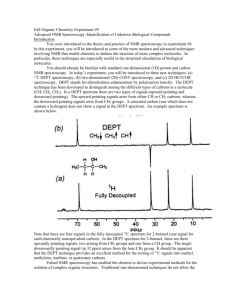
The 1 H spectrum and 13 C spectra for a particular compound are different. Below is a 13 C spectrum of ethanol.
The carbon-13 NMR shows two peaks representing the two carbon atom environments.
Summarising:
Analysis of an NMR spectrum involves consideration of: o The number of peak sets o The chemical shift of each peak set o The relative area under each peak set o Peak splitting.
Questions: 17, 18, 32 & 40.