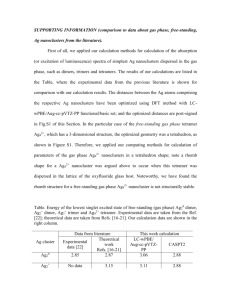
Finding a precise structure of gold nanoclusters will help our
advertisement

Rajiv Thakrar Tutor: D. Murtagh The audience of this article was aimed to be someone of a general science interest reading a newspaper. I looked through many articles in science, in sources such as in The Times and The Guardian, as well as reading the styles of New Scientist and Eureka Magazine. GOLD NANOCLUSTERS MAKE BIG ADVANCE In large amounts, gold is treasured as a non-reactive metal, where its use in electronics, dentistry, jewellery and art rely on its inertness. But recent discoveries by scientists at the Nanoscale Physics Laboratory, School of Physics and Astronomy have shown that nanoclusters of gold, made up of only a small number of atoms, exhibit chemical reactivity, making them powerful catalysts. The discovery that nanoscale gold particles function as active and selective catalysts for many important chemical reactions has provoked much interest in recent years. The physical and chemical properties of gold depend greatly on their precise physical structures. A significant effort has since been made by researchers to find an accurate configuration of gold nanoclusters. Detailed imaging will provide a new insight on this topic, and will open the door for similar investigations involving different metal particles of similar sizes. With this information, scientists would be able to find the most stable arrangements of gold clusters in specific size ranges. This is of great importance for revealing the chemical properties of these clusters and how they were put together. However, clear-cut determination of the three-dimensional structure of nanoparticles is challenging. The main process used for imaging at this scale is known as electron tomography, which involves firing an electron beam at a target and using the scattered electrons to build up an image of the structure of the particle. The mechanisms involved are similar to the light microscopes we have probably all used some time during out childhood, where the image we see is formed when light travels off the source and is focused in the lens. Generally, the approach is ideal for stable and stationary structures, but ultrasmall nanoparticles are structurally unstable, meaning that typical electron beams are simply too powerful to create images without destroying the formation of the atoms within the cluster. This is a pain for researchers, as finding the specific structure of nanoclusters may be essential for the progression of science at smaller scales. New techniques, published in Nature magazine, are set to revolutionise the way we see things. Using a new way of collecting data with weaker electron beams, scientists are able to create images of small particles without destroying their internal structure. Professor Zi You Li, of the Nanoscale Physics Laboratory, helped create this technique. He said that this new detection method “is the first detection technique in which rows of single atoms are seen, which gives a practical advantage over others. Being able to detect the structure of atoms in a cluster without damaging its structure or stability is something we have never been able to achieve before.” The technique uses various simple imaging simulations to determine the three-dimensional shape, orientation and atomic arrangement of size-selected gold nanoclusters. The geometric shapes of the gold nanoclusters can be determined for larger clusters, with over 30,000 atoms in them. High-resolution images of gold clusters on a carbon film. Typical images show various outline shapes, that is, cluster projections: pentagon (a), square (b) and hexagon (c). Scientists will be able to use this information to study the specific structures of gold and similar metals, which may be able to provide further insight of the roles of ultrasmall particles in nature.