Waves, Photons & the EM Spectrum
advertisement

Waves, Photons & the EM Spectrum
Outline
1. Astronomical information via em radiation
* EM radiation: wave and particle properties
2. Waves
* Transverse, Longitudinal
* Wavelength, amplitude, frequency
* Wavelength & frequency inversely related
3. Electromagnetic Waves
* Oscillating electric/magnetic field
* Originate in acclerated charge
* Travel through empty space
* Travel at speed of light (in vacuum)
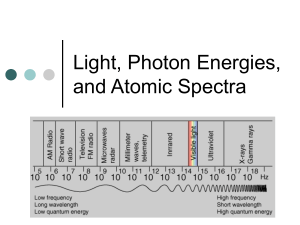
4. Electromagnetic Spectrum
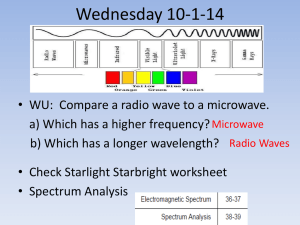
* Span of all visible wavelengths
* Visible light only small part ('ROY G. BIV')
5. Photons
* E a f, E a 1/l
6. Interaction of Light & Matter
* Emission, Absorption, Transmission, Reflection
7. Spectra
* Light dispersed into its constituent wavelengths
* Emission line, absorption line, continuous
* Emission Line
> Bright lines on dark background
> Distinctive pattern for each element
* Absorption Line
> Dark lines on bright background
> Distinctive pattern for each element
* Emission/Absorption line produced when electrons hop between energy levels in atom
* Continuous
> Continuous band of color
> Blackbodies
- Distinctive curve of blackbody radiation
- amt of energy emitted a Temp4
- peak of BB curve shifts to short wavelength as temp increases
> Blackbodies & Color
* Spectrum of Mars
8. Doppler Effect
* Change in observed wavelength/frequency due to radial motion of source of waves
* Light: shift of wavelength of spectrum lines: blueshift/redshift
Questions
1. What is meant by the frequency of a wave? How is frequency related to wavelength?
2. What property of the different colors of light causes us to perceive them differently?
3. Consider waves having wavelength 2.0 m and 6.0 m:
a) Which has the higher frequency?
b) Which has the longer period?
4. Briefly explain your answers to the following. Which is greater:
a) the speed in vacuum of an x-ray photon or of a radio photon?
b) the frequency of an x-ray photon or of a radio photon?
c) the energy of an x-ray photon or of a radio photon?
5. Why do you suppose that ultraviolet light can cause skin cancer but ordinary visible light
cannot?
6. Explain how the spectrum of hydrogen is related to the structure of the hydrogen atom.
7. Why do different chemical elements have different patterns of lines in their spectra?
8. An imaginary atom has just 4 energy levels: 0 eV, 1 eV, 3 eV and 6 eV. (The eV, or electron
volt, is a unit of energy.) Here’s an energy level diagram for this atom:
6
3
Energy
(eV)
1
0
Draw all possible transitions between energy levels. For which transition is the associated
photon energy largest? smallest? For which transition is the associated photon wavelength
longest? shortest?
9. A star the having the same radius as the Sun, but with twice the Sun’s temperature would
radiate how much more energy than the Sun? If the temperature were one-third the Sun’s
temperature, how much energy would be emitted?
10. The line spectra depicted below arise in stars (A and C) and a stationary source (B). (These
are artificially simple spectra, consisting as they do of only three lines.)
a) Which of the stars A and C is moving toward Earth? Away from Earth?
b) Which of the stars A and C has the greater speed (say, in km/s)? How can you tell?
c) Were this an emission line spectrum, what would you expect to be the color of the line on the
far right?
Answers
1. The frequency of a wave is the number of wave (actually, wavelengths) that pass a point per
second. Frequency and wavelength are inversely related: higher frequency means shorter
wavelength; lower frequency means longer wavelength.
2. Eye and brain associate color with different wavelengths of light.
3. a) The 2.0 m wavelength wave has higher frequency.
b) Period is the time required for a wave to pass a point (it's the reciprocal of frequency). So,
longer wavelength means longer period. Thus, the 6.0 m wavelength wave would have the
longer period.
4. a) Speeds are equal (300,000 km/s). All electromagnetic waves, regardless of wavelength,
travel at the same speed in vacuum.
b) The frequency of the x-ray photon is greater because its wavelength is shorter than that of the
radio photon.
c) The energy of an x-ray photon is higher. The energy carried by a photon is directly
proportional to its frequency: The higher the frequency, the greater the energy carried by the
photon.
5. The individual photons of UV light carry more energy than individual photons of visible light.
More energy means greater potential for damage to skin tissue.
6. Have a look at Fig. 6.6 on pg. 102 in your textbook. Each spectrum line falls at a specific
wavelength; each wavelength corresponds to a particular amount of energy carried by each
photon at that wavelength. And the amount of energy carried by each photon corresponds to a
gap between electron energy levels in an atom, in this case the hydrogen atom. So, measuring
the wavelengths of the spectrum lines allows us to determine how the electron energy levels are
arranged in the atom. As each atom's spectrum is unique, so too is its arrangement of energy
levels.
7. Different arrangements of energy levels naturally result in different patterns of spectrum lines.
(See answer to Ques. #6.)
8.
6
3
Energy
(eV)
1
0
As you can see, there are 6 possible transitions (pathways) connecting energy levels. Photon
energy is largest for 0 eV – 6 eV transition (3rd from left). Photon energy is smallest for the
0 eV – 1 eV transition. In any case, the energy of the photon associated with a transition is equal
to the difference in energy between the energy levels. Wavelength () is longest for the 0 eV – 1
eV transition, because energy and wavelength are inversely proportional:
1
E
Using the same reasoning, we see that the shortest wavelength photon is associated with the 0 eV
– 6 eV transition.
9. Stars radiate approximately like black bodies, so we can use one of the “rules for black
bodies” to solve this problem:
Amt. of energy emitted
from each sq. meter
T 4
In this case, as the two stars have equal surface areas, comparing the amount of energy emitted
from each square meter is identical to comparing the amounts of energy emitted from the two
stars. For a star twice the Sun’s temperature:
4
2
16
1
So, such a star would radiate 16 times as much energy as the Sun. As for a star with 1/3 the
Sun’s temperature:
4
4
1
1/3
1
81
1
3
So, such a star would radiate only 1/81 as much energy as the Sun (!).
10. a) Star A is moving toward Earth, as its spectrum shows a blueshift. Star C is moving away
from Earth, as its spectrum shows a redshift.
b) Star C has the greater speed as its spectrum lines show a larger amount of shift than do the
lines of Star A.
c) The line on the far right is each spectrum would most likely be red in color, as the longest
wavelengths in the visible spectrum correspond to the color red.