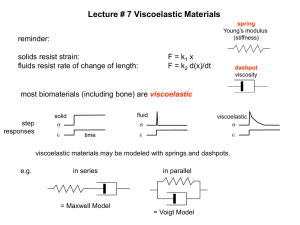
Measurement of Viscoelastic Response Using Torsional Waves
advertisement

Viscoelastic Response of Vocal Fold Tissues and Scaffolds at High Frequencies R. J. Clifton,1 X. Jia,2 T. Jiao1, C. Bull1, M. S. Hahn,3 1 Division of Engineering, Box D, Brown University, Providence, RI, ,02912, USA clifton@engin.brown.edu, Tong_Jiao@brown.edu, Christopher_Bull@brown.edu 2 Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., E25/342, Cambridge, MA 02139, USA xinqiao@mit.edu 3 Department of Chemical Engineering, Texas A&M University, 3122 TAMU, College Station, TX, 77843-3122, USA mariah.hahn@chemail.tamu.edu Abstract A new experimental capability is presented for measuring the viscoelastic properties of vocal folds and candidate scaffold materials at phonation frequencies. Thin cylindrical samples are subjected to torsional wave loading by using a high frequency galvanometer to oscillate a plate at the base of the sample. The rotation of the base plate and a top plate are recorded using optical levers. Viscoelastic properties of the sample are determined by fitting the amplification of the motion of the top of the sample to that predicted by a linear viscoelastic wave analysis. Results are reported for canine and ferret vocal folds as well as for hyaluronic acid (HA)-based hydrogels. 1 Introduction During normal speech, human vocal folds sustain more than 100 high impact collisions each second. Voice overuse may generate nodules on the outer layer of the vocal folds ─ the so-called superficial lamina propria (SLP). In other cases, pathological conditions may render part of the tissue cancerous. In any case, when damaged tissue is removed by surgery, the resulting scar tissue lacks the pliability of the original tissue and voice quality is often seriously reduced. Over the past few years, significant research effort has been directed toward using tissue engineering approaches to regenerate vocal fold vibratory tissue that responds as normal LP. As a first step toward developing suitable replacement materials it is important to understand the mechanical response of the natural tissue that they will replace, as well as their own mechanical response at frequencies of human phonation ─ approximately 100 – 900 Hz. Chan and Titze (1999, 2000) measured the viscoelastic properties of human vocal folds by subjecting LP tissue to torsional oscillations in a parallel-plate rheometer. From these experiments they obtained the frequency-dependent storage (shear) modulus and viscosity of the LP over frequencies ranging from 0.01 to 10 Hz. Measurements at 15 Hz did not follow the trend lines of the results at lower frequencies and were deemed, by the authors, to be ‘marginally acceptable’. 1 An important conclusion from the Chan and Titze (1999, 2000) experiments is that the shear modulus of human vocal folds is very low. For most subjects the shear modulus G1 at a frequency of 10 Hz ranges from approximately 10 to 100 Pa. The elastic shear wave speed cs G1 / for a material with a shear modulus G1 100 Pa and a density = 1000 kg/m3 is approximately 30 cm/s. Because this wave speed is so small relative to wave speeds in most solid materials, one can expect that strong limitations will be imposed on the maximum frequency for which the mechanical properties of the sample can be inferred by means of the usual interpretation of rheometric tests ─ based on assuming that the stress is nominally uniform through the thickness of the sample. For solid samples the latter assumption holds when the time required for roundtrip transit of stress waves through the thickness of the sample is much less than the period of a single oscillation, i.e. for f cs 2h (1.1) where h is the sample thickness and f is the driving frequency. For human LP with a thickness h=0.03cm, as used by Chan and Titze (1999, 2000), the limitation (1.1) becomes f<<500 Hz or, say, f<30 Hz. Even lower limits on allowable frequencies are obtained for samples with shear moduli near the lower limits of the range of measured values. Analogously, for fluid samples, a requirement that stresses due to sample inertia are small relative to those due to sample viscosity leads to the limitation (e.g. Schlichting, 1960) f 2 2 h 2 (1.2) where is the viscosity of the sample. From viscosity measurements of Chan and Titze (1999, 2000) the frequency dependent viscosity of human LP can be described approximately by 0 f 0.85 with 0 1.0Pa s . Substitution of this expression for into (1.2) gives the limitation f<<83 Hz or, say, f<10 Hz. Whether the frequency limitation is obtained from a constraint of type (1.1) or (1.2) it appears that the frequencies for which the viscoelastic properties of human LP can be measured by standard rheometric methods are likely to be below the frequencies of phonation. Other methods for measuring viscoelastic properties at high frequencies include electromagnetic torsion methods (Brodt, et al., 1995), electromechanical tensile test methods (Hemler et al., 2001), and stress-controlled rheometer methods at low audio frequencies (Titze et al., 2004). While all of these methods have attractive features they all neglect wave propagation in the sample. Consequently, they all have frequency limitations similar to those of (1.1) and (1.2), although the upper limit on allowable frequencies may be extended by using smaller samples. An alternative approach, based on the analysis of longitudinal waves in viscoelastic cylindrical rods, has been introduced by Jia et al. (2004) to determine the viscoelastic properties of photo-cross-linked hydrogels. An acoustic shaker was used to subject impose the base of the rod to an oscillatory vertical motion. The motion of the free, top end of the rod was monitored using a laser-Doppler vibrometer. From the measured 2 amplification factor (the amplitude of the velocity at the top of the rod divided by the amplitude of the velocity at the shaker surface), and the phase shift between the top and bottom ends of the rod, the wave propagation solution was used to determine the frequency dependent viscoelastic moduli for the hydrogel. While this method could extend the measurement of viscoelastic properties into the range of phonation frequencies, it could not be used for LP because the LP geometry does not allow the preparation of slender cylindrical specimens. In the present paper we retain the basic ideas of the method of Jia et al. (2004), but modify the configuration to be that of torsional waves in a cylindrical sample. With this modification it becomes possible to measure viscoelastic properties of LP and of candidate replacement materials at phonation frequencies. Furthermore, because small amplitude torsional waves are described exactly by a one dimensional wave theory the interpretation of the experiments is free of such complications as geometric dispersion and lateral inertia effects that encumber the interpretation of experiments involving longitudinal wave propagation in rods. In the pilot experiments described here, results are reported for canine LP, ferret LP, and a number of hyaluronic acid (HA)-based hydrogels. These hydrogel networks exhibit prolonged in vivo residence time, while providing a range of viscoelastic properties that span the range of responses observed for LP tissue. These hydrogels are expected to be biocompatible and to be favorable for promoting cell growth. 2 Torsional Wave Analysis Consider the propagation of torsional waves in a cylindrical, viscoelastic sample with its axis lying along the z-direction. The torsional moment or torque at position z at time t can be represented as a T z , t 2 r 2 z , r , t dr (1.3) 0 where a is the radius of the sample and ( z , r , t ) is the shear stress corresponding to the strain rate history z, r , t / t according to (e.g. Ferry, 1980) t z, r , t G t t ' 0 z , r , t ' dt ' t ' (1.4) where G(t) is the viscoelastic relaxation modulus in shear. Assume that the deformation within the sample can be described adequately as pure torsion in which planes of constant z rotate about the axis of symmetry by an amount (z,t) so that the condition of kinematical compatibility becomes z, r , t ' v z, r , t ' z , t ' r t ' z z (1.5) where v(z,r,t’) is the (circumferential) particle velocity. Substitution of (1.5) and (1.4) 3 into (1.3) gives t T z, t J G t t ' z, t ' z 0 dt ' (1.6) where J a4 (1.7) 2 is the polar moment of inertia of the cross-sectional area and z, t z , t t (1.8) is the angular velocity of the cross-section. Consideration of the rate of change of angular momentum for a circular slice of the sample gives the dynamical equation J z, t T z , t t z (1.9) where is the mass density of the sample material. Substitution of (1.6) into (1.9) gives the following integral-partial-differential equation for the angular velocity (z,t). z, t t 2 z, t ' G t t ' dt ' t z 2 0 (1.10) 2.1 Solution We seek a solution of (1.10) that vanishes for t<0 and matches measured angular velocities at the two ends of the sample, say z=0 and z=h. More specifically, the objective here is to find the relaxation function G(t) that corresponds to measured angular velocities 0(t) at z = 0 and h(t) at z = h. Just as the direct problem requires an additional boundary condition at z = h to predict the motion h(t) from the motion 0(t) and the function G(t), the determination of G(t) from 0(t) and h(t) requires an additional boundary condition at z = h. This additional boundary condition expresses the nature of the restraint at z = h. For interpretation of experimental results on a cylindrical sample sandwiched between two rigid plates we consider the cylindrical sample to be attached to a rigid plate at z = h. The rigid plate is taken to have density 0 and polar moment of inertia I0. The plate is assumed to be bonded to the cylindrical viscoelastic sample at z = h, but is otherwise free. The dynamical equation for the angular acceleration of the rigid plate gives the following boundary condition at z = h. t J G t t ' 0 z , t ' z z h dt ' 0 I 0 4 h, t t (1.11) To solve Eqns. (1.10) and (1.11) we take the Laplace transform of the field equation (1.10) and the boundary condition (1.11) to obtain 2 z, s s z , s G s z 2 and JG s h, s 0 I 0 s 2 h, s z (1.12) (1.13) respectively, where a superposed ~ denotes the Laplace transform of the underlying ~ ~ function. Replacement of by s in (1.12) and (1.13) gives the ordinary ~ differential equation for z, s , s z , s G s 2 z , s z 2 (1.14) and the boundary condition, JG s h, s 0 I 0 s h, s . z (1.15) Equation (1.14) has a solution of the form, z, s A cosh z B sinh z (1.16) where ~ s s / G s (1.17) and the coefficients A and B are to be determined from the boundary condition (1.15) and the imposed motion at z=0. We assume that the imposed motion is the harmonic motion 0, t 0 exp i0t (1.18) where 0 and 0 are constants. The coefficient A obtained by the substitution of (1.16) into the Laplace transform of (1.18) is A s 0 s i0 (1.19) 5 From the boundary condition (1.15), and the relation (1.19), the coefficient B is ~ B J G s s sinh s h 0 I 0 s cosh s h 0 . s i 0 J G s s cosh s h 0 I 0 s sinh s h ~ (1.20) ~ To invert the Laplace transform h, s we use the definition of the inverse transform: h, t i ~ 1 exp st h, s . 2 i i (1.21) The parameter is a positive real number so the integration path is to the right of the imaginary axis in the s-plane. The integration contour can be closed in the left half plane. No contribution is obtained from the contour at . Therefore, by the Residue Theorem, the integral (1.21) is simply equal to 2i times the sum of the residues of the integrand at poles to the left of s= plus possible contributions from integrals along branch cuts. The only pole is at s=i 0. Here i0 i0 ~ G i0 02 i G * 0 (1.22) in which (e.g. Ferry, 1980) ~ G * 0 i0 G i0 (1.23) is the complex modulus for the viscoelastic response of the sample material in simple shear at circular frequency 0. Characterization of the viscoelastic response can be put into more readily interpretable form by representing the complex modulus in terms of its magnitude |G*( )| and its phase angle (): G * G * e i . (1.24) In writing the solution it is helpful to simplify the argument to h i i (1.25) where 0 h 0 0 , cos , sin . G * 0 2 2 Then, the angular velocity at the end z = h can be written in the form h, t M 0 0 cos 0t 0 6 (1.26) in which M(0 ) is the amplification factor: M c (c cos( ) d sin( )) 2 (c 2 d 2 )sinh 2 ( ) c cos d sin( ) c 2 2 d 2 sinh 2 where c c 0 J G * 0 d d 0 0 I 00 and 0 is the phase shift characterized by tan 0 If the response c sin d cos sinh . c cos d sin cosh M 0 , 0 is measured over a range of frequencies, then the moduli |G*( 0)|, (0) can be estimated by means of a regression analysis. Parameter values in the regression models are readily obtained using an Excel spreadsheet and minimizing the differences between calculated and measured values by using the Solver routine. 3 Experimental Setup The experimental setup is shown in Figure 1. A thin cylindrical sample of a soft material is placed between two hexagonal plates. The bottom plate is rotated back and forth through angles up to 6o by means of an optical scanner (GSI Lumonics, Model 000-G112) at frequencies up to 2500 Hz. To ensure that the shear strains in the sample are sufficiently small for linear viscoelasticity to be a satisfactory approximation, the imposed rotations are kept less than 0.2o. The rotations of the top and bottom plates are monitored by an optical lever technique in which laser beams, reflected off aluminized faces of the hexagonal plates, are passed through a spherical lens, a cylindrical lens and a butterfly-shaped aperture before being captured by photodiode detectors. The spherical lens expands the beam and the cylindrical lens focuses the beam into a vertical sheet of light at the aperture. The spherical lens is selected to ensure that the height of the sheet of light at the aperture is greater than the maximum height of the aperture. Because of the shape of the aperture (See End View in Figure 1) the light power collected, and consequently the output of each photodiode, is proportional to the angular rotation of the respective plate. The rotation of the bottom plate is driven by a computer-controlled frequency generator that steps through a sequence of frequencies from a minimum frequency, fmin, to a maximum frequency, fmax, in steps f . At each frequency the amplitude of the rotation of each plate is obtained as the average amplitude over a fixed number of cycles, usually 10, after transients from the change in frequency have died out. The output of the photodiodes is monitored by an oscilloscope (Agilent 54622A) that provides directly the average amplitude of the signal, less its minimum value. The difference between these two 7 quantities is proportional to the rotation of the respective plate. The experimentally determined amplification factor is obtained as the ratio of the amplitude of the rotation of the top plate divided by that of the bottom plate. Calibration differences between the recorded outputs for the two plates are accounted for by adjusting the experimentally determined amplification factor to approach the required value of unity as the frequency goes to zero. Incident Beam f1 Mask f2 Object Plane Sample Spherical Lens Top View Image Plane Photodiode Detector Cylindrical Lens Mask End View Side View Fig. 1. Optical layout for torsional wave experiments Amplification factors for each frequency, determined from the experimental records as described above, are compared with those predicted by the viscoelasticity model described in the previous section. For that model the viscoelastic description of the material is expressed in terms of the amplitude of the complex shear modulus, |G*( )|, and the loss angle, (). The height and diameter of the sample, required for the wave analysis, are obtained from digital images of the sample in place. The output signal shows a peak in the amplification factor at a frequency, fpeak.. For each test, a constant modulus |G*| and phase shift are obtained that provide the best fit between the model and the experimental results over a range of frequencies spanning the frequency, fpeak. 8 4 Results and Discussion An example of results for HA-based hydrogels is shown in Figure 2. The cylindrical samples were punched out of sheets, synthesized in a flat-bottomed container and cross-linked using a UV light source. Tests were conducted under conditions of ambient temperature and humidity. Close agreement between the results of the experiment and the predictions of the model for constant values of the viscoelastic parameters (G1,) suggests the validity of the model and the relatively weak dependence of these parameters on frequency over a range of frequencies comparable 18.00 16.00 a Dimensionless 14.00 b c 12.00 10.00 8.00 6.00 4.00 2.00 0.00 0 50 100 150 200 Frequency, Hz Fig. 2. Frequency dependence of amplification factors for three synthetic hydrogels; curves are model fits and symbols are experimental results. Hydrogel compositions and the viscoelastic moduli that provide the best fit between model predictions and experimental results are: (a) HAGMA (2%), h=0.969 mm, a = 2.52 mm, G1=1514 Pa, δ=0.127 radians. (b) HAGMA (2%)/ PEGDA[MW=575](6%), h=0.997 mm, a=2.54 mm,G1=3230 Pa, δ=0.132 radians. (c) HAGMA (2%) PEGDA[MW=2000](12%), h=0.969mm, a=2.51mm, G1=6066 Pa δ=0.14 radians. Inset shows the sample (c) between the two plates of the loading system. PEGDA: polyethylene glycol diacrylate; HAGMA: Glycidyl methacrylate modified hyaluronic acid; [MW]: molecular weight. 9 250 to the widths of the peaks, say 50 Hz. The increase in modulus with increasing fractions of PEGDA is consistent with expectations of the stiffening effect of this additive. Results are repeatable for successive tests on the same sample as long as the time between tests is not so long that the moisture content of the sample changes significantly. Results are also reproducible for separate samples prepared in the same way. Examples of results for lamina propria samples taken from a dog larynx and from a ferret larynx are shown in Figure 3. Samples were prepared by separating the LP from the vocalis muscle and then punching out a cylindrical sample with a thickness equal to the full thickness of the LP. Again the tests were conducted under conditions of ambient temperature and humidity. The fit between model and experiment is not as good as that shown in Figure 2 for hydrogels. Better agreement can be obtained by including weak dependence of the viscoelastic moduli on frequency. Nevertheless, the agreement is sufficiently good to give confidence in the approximate levels of the complex modulus characterized by the parameters (G1,). Much lower peak values for the amplification factors result in much larger values for the loss angles . 4.00 Dimensionless 3.50 3.00 Ferret LP 2.50 Dog LP 2.00 1.50 1.00 0.50 0.00 0 20 40 60 80 100 120 140 Frequency (Hz) Fig. 3. Amplification factors for lamina propria samples obtained from a dog and a ferret; curves are model fits and symbols are experimental results. Sample dimensions and the values of the viscoelastic moduli that provide the best fit to the experimental measurements are as follows: Dog LP: h= 0.371 mm, a=1.74 mm, G1=1423 Pa, δ=0.898 radians; Ferret LP: h= 0.27 mm, a=1.43 mm, G1=1944 Pa, δ=1.131 radians. Inset shows the dog LP between the two plates of the loading system. 10 Acknowledgments. RJC is grateful to Brown University for Sabbatical Leave support that made this research possible as well as to Bob Langer at MIT and Steven Zeitels at Massachusetts General Hospital for giving him the opportunity to join their research effort on vocal fold regeneration. We thank James Kobler for his help with tissue processing. XJ and MSH are grateful to the Eugene B. Casey Foundation and the Advisory Board Foundation for their financial support. References Chan, R.W., and Titze, I.R. (1999) Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results, J. Acoust. Soc. Am. 106: 2008-2021. Chan, R.W., and Titze, I.R. (2000) Viscoelastic shear properties of human vocal fold mucosa: theoretical characterization based on constitutive modeling, J. Acoust. Soc. Am. 107: 565-580. Schlichting, H. (1960) Boundary Layer Theory, 4th Ed.,, (Translated by J. Kestin), McGraw-Hill, NewYork. Brodt, M., Cook, L.S., and Lakes, R.S. (1995) Apparatus for measuring viscoelastic properties over ten decades: refinements, Review of Scientific Instruments 66: 5292-5297. Hemler, R.J., Wieneke, G.H., van Riel, A.M., Lebacq, J., and Dejonckere, P.H. (2001) A new method for measuring mechanical properties of laryngeal mucosa, Eur. Arch. Otorhinolaryngol 258:130-136. Titze, I.R., Klemuk, S.A., and Gray, S. (2004) Methodology for rheological testing of engineered biomaterials at low audio frequencies, J. Acoust. Soc. Am. 115: 392401. Jia, X., Burdick, J.A., Kobler, J., Clifton, R.J., Rosowski, J.J., Zeitels, S.M., and Langer, R. (2004) Synthesis and characterization of in situ crosslinkable hyaluronic acid-based hydrogels with potential application for vocal fold regeneration, Macromolecules 37: 3239-3248. Ferry, J.D. (1980) Viscoelastic Properties of Polymers, 3rd Ed. , Wiley and Jones, New York. 11 12