AP_Physics_Assignments_files/AP Lesson 16 HW Key
advertisement
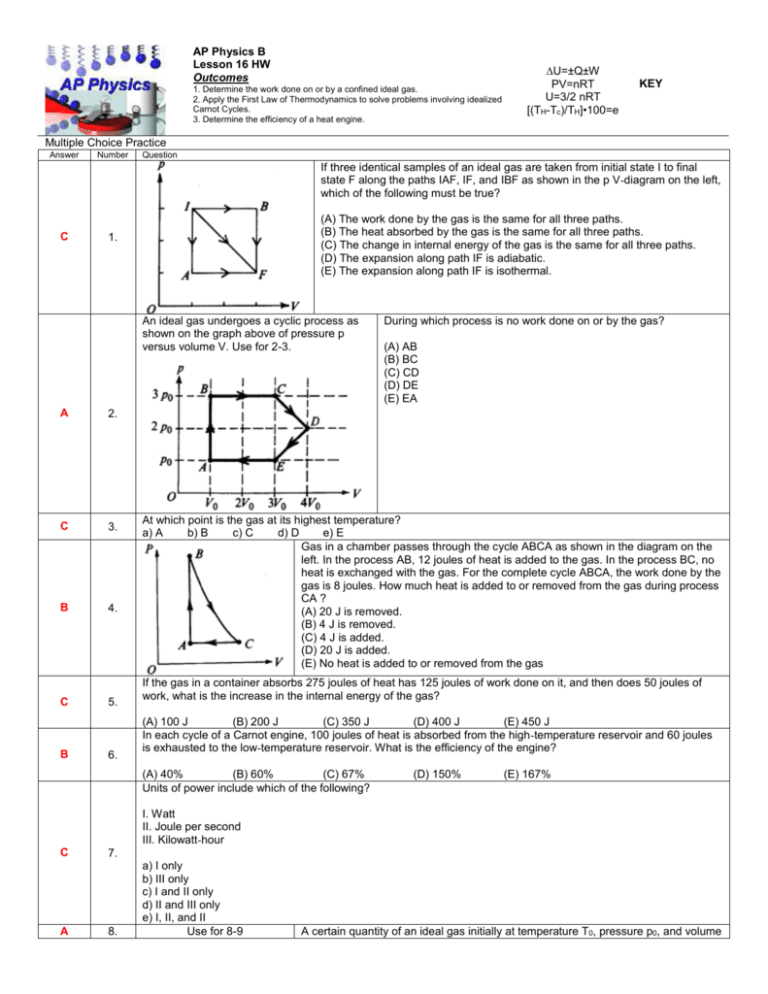
AP Physics B Lesson 16 HW Outcomes 1. Determine the work done on or by a confined ideal gas. 2. Apply the First Law of Thermodynamics to solve problems involving idealized Carnot Cycles. 3. Determine the efficiency of a heat engine. ∆U=±Q±W PV=nRT U=3/2 nRT [(TH-Tc)/TH]•100=e KEY Multiple Choice Practice Answer Number Question If three identical samples of an ideal gas are taken from initial state I to final state F along the paths IAF, IF, and IBF as shown in the p V-diagram on the left, which of the following must be true? C (A) The work done by the gas is the same for all three paths. (B) The heat absorbed by the gas is the same for all three paths. (C) The change in internal energy of the gas is the same for all three paths. (D) The expansion along path IF is adiabatic. (E) The expansion along path IF is isothermal. 1. An ideal gas undergoes a cyclic process as shown on the graph above of pressure p versus volume V. Use for 2-3. A 2. C 3. B 4. C B 5. 6. During which process is no work done on or by the gas? (A) AB (B) BC (C) CD (D) DE (E) EA At which point is the gas at its highest temperature? a) A b) B c) C d) D e) E Gas in a chamber passes through the cycle ABCA as shown in the diagram on the left. In the process AB, 12 joules of heat is added to the gas. In the process BC, no heat is exchanged with the gas. For the complete cycle ABCA, the work done by the gas is 8 joules. How much heat is added to or removed from the gas during process CA ? (A) 20 J is removed. (B) 4 J is removed. (C) 4 J is added. (D) 20 J is added. (E) No heat is added to or removed from the gas If the gas in a container absorbs 275 joules of heat has 125 joules of work done on it, and then does 50 joules of work, what is the increase in the internal energy of the gas? (A) 100 J (B) 200 J (C) 350 J (D) 400 J (E) 450 J In each cycle of a Carnot engine, 100 joules of heat is absorbed from the high-temperature reservoir and 60 joules is exhausted to the low-temperature reservoir. What is the efficiency of the engine? (A) 40% (B) 60% (C) 67% Units of power include which of the following? (D) 150% (E) 167% I. Watt II. Joule per second III. Kilowatt-hour C A 7. 8. a) I only b) III only c) I and II only d) II and III only e) I, II, and II Use for 8-9 A certain quantity of an ideal gas initially at temperature T0, pressure p0, and volume V0 is compressed to one-half its initial volume. As shown above, the process may be adiabatic (process 1), isothermal (process 2), or isobaric (process 3). Which of the following is true of the mechanical work done on the gas? a) It is greatest for process 1. b) It is greatest for process 3. c) It is the same for processes 1 and 2 and less for process 3. d) It is the same for processes 2 and 3 and less for process 1. e) It is the same for all three processes. Which of the following is true of the final temperature of this gas? A 9. a) It is greatest for process 1. b) It is greatest for process 2. c) It is greatest for process 3. d) It is the same for processes 1 and 2. e) It is the same for processes 1 and 3. In a certain process, 400 J of heat is added to a system and the system simultaneously does 100 J of work. The change in internal energy of the system is C 10. a) 500 J b) 400 J c)300 J d)-100 J e) -300 J An ideal gas is initially in a state that corresponds to point 1 on the graph above, where it has pressure p1, volume V1, and temperature T1. The gas undergoes an isothermal process represented by the curve shown, which takes it to a final state 3 at temperature T3. If T2 and T4 are the temperatures the gas would have at points 2 and 4, respectively, which of the following relationships is true? B 11. A 12. C 13. C 14. a) T1 < T3 b) T1 < T2 c) T1 < T4 d) T1 = T2 e) T1 = T4 A gas with a fixed number of molecules does 32 J of work on its surroundings and 16 J of heat are transferred from the gas to the surroundings. What happens to the internal energy of the gas? (A) It decreases by 48 J. (B) It decreases by 16 J. (C) It remains the same. (D) It increases by 16 J. (E) It increases by 48 J. Use for 13-14. For the process X Y, U is greater than zero and a) Q<0 and W=0 b) Q<0 and W>0 c) Q>0 and W<0 d) Q>0 and W=0 e) Q>0 and W>0 A thermodynamic system is taken from an initial state X along the path XYZX as shown in the PV-diagram above. For the process YZ, Q is greater than zero and a) W<0 and U=0 b) W=0 and U<0 c) W=0 and U>0 d) W>0 and U=0 e) W>0 and U>0 Free Response Practice 1975B3. One mole of a monatomic ideal gas enclosed in a cylinder with a movable piston undergoes the process ABCDA shown on the p-V diagram above. a. In terms of po and Vo calculate the work done by the gas in the process. W=∆P•∆V = 2Po•2Vo = 4PoVo b. In terms of po and Vo calculate the net heat absorbed by the gas in the process. Net W = Net Q = 4PoVo c. At what two lettered points in the process are the temperatures equal? Explain your reasoning. B & D PV=nRT 3PoVo = Po3Vo 1979B5. Four samples of ideal gas are each initially at a pressure Po and volume Vo, and a temperature To as shown on the diagram above. The samples are taken in separate experiment from this initial state to the final states I, II, III, and IV along the processes shown on the diagram. a. One of the processes is isothermal. Identify which one and explain. Process II is isothermal. If T is constant, Po/Vo = (Po/2)(2Vo) b. One of the processes is adiabatic. Identify this one and explain. Process III. P and V must both change in an adiabatic process. This condition eliminates process I and IV. Process II is isothermal so the only remaining possibility is III. c. In which process or processes does the gas do work? Explain. I, II, III. Work is done by the gas when the volume increases. d. In which process or processes is heat removed from the gas? Explain. Heat is removed in process IV. Pressure decreases at a constant volume. This means U decreases, Q has to be remove for this to occur at constant volume. e. In which process or processes does the root-mean-square speed of the gas molecules increase? Explain. I. The temperature increases in process I, so the V rms must go up. T is directly proportional to KE which depends on Vrms. 1986B5. A proposed ocean power plant will utilize the temperature difference between surface seawater and seawater at a depth of 100 meters. Assume the surface temperature is 25° Celsius and the temperature at the 100-meter depth is 3° Celsius. a. What is the ideal (Carnot) efficiency of the plant? [(Th-Tc)/(Th)]•100 = e = [(298-276)/(298)]100=7.4% b. If the plant generates useful energy at the rate of 100 megawatts while operating with the efficiency found in part (a), at what rate is heat given off to the surroundings? 100MW/0.074 = 1351 MW Total 1351MW-100MW=1250MW Exhausted. c. A nuclear power plant operates with an overall efficiency of 40 percent. At what rate must mass be converted into energy to give the same 100-megawatt output as the ocean power plant above? Express your answer in kilograms per second. 100MW/0.4 = 250 MW E=mc2 E/c2 =m c = speed of light = 3x108 m/s. 250,000,000/(3x108 m/s)2 = 0.83 to reactor or 0.33kg/s for just net output. The diagram below represents the Carnot cycle for a simple reversible (Carnot) engine in which a fixed amount of gas, originally at pressure po and volume Vo follows the path ABCDA In the chart below, for each part of the cycle indicate with +, -, or 0 whether the heat transferred Q and temperature change T are positive, negative, or zero, respectively. ( Q is positive when heat is added to the gas, and T is positive when the temperature of the gas increases.) 1989B4. An ideal gas initially has pressure po, volume Vo, and absolute temperature To. It then undergoes the following series of processes: I. It is heated, at constant volume, until it reaches a pressure 2po. II. It is heated, at constant pressure, until it reaches a volume 3 Vo. III. It is cooled, at constant volume, until it reaches a pressure po. IV. It is cooled, at constant pressure, until it reaches a volume Vo. a. On the axes below i. Wnet = enclosed area w=∆P•∆V= 2PoVo i. draw the p-V diagram representing the series of ii. ∆U=0 for cycle processes; iii. Net Q= net W = 2PoVo ii. label each end point with the appropriate value of absolute temperature in terms of To. b. For this series of processes, determine the following in terms of po and Vo. i. The net work done by the gas ii. The net change in internal energy iii. The net heat absorbed c. Given that Cp = (5/2)R and Cv = (3/2)R, determine the heat transferred during process 2 in terms of Po and Vo. Q=nCp∆T = 5/2 nR∆T = 5/2nR4To = 10nRTo PV=nRT nRTo=PoVo so with substitution Q= 10PoVo PV/nR=T a) [(1x105 Pa)(25x10-3m3)/[(1)(8.314J/mol•K)] = T = 300K b) [(2x105 Pa)(25x10-3m3)/[(1)(8.314J/mol•K)] = T = 600K c) [(1x105 Pa)(50x10-3m3)/[(1)(8.314J/mol•K)] = T = 600K 1990B4. One mole of an ideal monatomic gas, initially at point A at a pressure of 1.0 x 105 newtons per meter squared and a volume of 25 x l0-3 meter cubed, is taken through a 3-process cycle, as shown in the pV diagram above. Each process is done slowly and reversibly. For a monatomic gas, the heat capacities for constant volume and constant pressure are, respectively, Cv = (3/2)R and Cp = (5/2)R, where R is the universal gas constant, 8.32 J/mole K. Determine each of the following: a. the temperature of the gas at each of the vertices, A, B. and C, of the triangular cycle above b. the net work done by the gas for one cycle Wnet = enclosed area = ½[∆P•∆V] = ½[(1x105Pa)(25x10-3m3)] = 1250 J c. the net heat absorbed by the gas for one full cycle Since ∆U for cycle is = 0, Wnet=Qnet = 1250J d. the heat given off by the gas for the third process from C to A Q = nCp∆T = (1mol)(5/2R)(600K-300K) = 6235 J e. the efficiency of the cycle e= Work out/ Qin = [1250J/ [ 6235J+1250J] ]100= 17% 1995B4. (10 points) A heat engine operating between temperatures of 500 K and 300 K is used to lift a 10-kilogram mass vertically at a constant speed of 4 meters per second. a. Determine the power that the engine must supply to lift the mass. P=F•v = N•m/s = Joules/second = (10kg)(10m/s2)(4m/s) = 400 J/s or 400 watts b. Determine the maximum possible efficiency at which the engine can operate. [(Th-Tc)/Th]100 = [(500K-300K)/500K]100=40% c. If the engine were to operate at the maximum possible efficiency, determine the following. i. The rate at which the hot reservoir supplies heat to the engine 400 watts / 0.4 = 1000 watts ii. The rate at which heat is exhausted to the cold reservoir 1000 watts-400 watts = 600 watts 1999B7 (10 points) A cylinder contains 2 moles of an ideal monatomic gas that is initially at state A with a volume of 1.0 x 10-2 m3 and a pressure of 4.0 x 105 Pa. The gas is brought isobarically to state B. where the volume is 2.0 x 10-2 m3. The gas is then brought at constant volume to state C, where its temperature is the same as at state A. The gas is then brought isothermally back to state A. a. Determine the pressure of the gas at state C. PaVa=PcVc Pc=Pa(Va/Vc) = [(4x105Pa)(1x10-2m3)]/2x10-2m3=Pc=2x105Pa b. On the axes below, state B is represented by the point B. Sketch a graph of the complete cycle. Label points A and C to represent states A and C, respectively. c. State whether the net work done by the gas during the complete cycle is positive, negative, or zero. Justify your answer. Positive – The enclosed area is positive work is done on the environment for the cycle. d. State whether this device is a refrigerator or a heat engine. Justify your answer. This is a heat engine that converts heat into useful work. The net heat added = net work done. 2001B6 (10 points) A cylinder is fitted with a freely moveable piston of area 1.20 x 10-2 m2 and negligible mass. The cylinder below the piston is filled with a gas. At state 1, the gas has volume 1.50 x 10-3 m3, pressure 1.02 x 105 Pa, and the cylinder is in contact with a water bath at a temperature of 0°C. The gas is then taken through the following four-step process. • A 2.50 kg metal block is placed on top of the piston, compressing the gas to state 2, with the gas still at 0°C. • The cylinder is then brought in contact with a boiling water bath, raising the gas temperature to 100°C at state 3. • The metal block is removed and the gas expands to state 4 still at 100°C. • Finally, the cylinder is again placed in contact with the water bath at 0°C, returning the system to state 1. a. Determine the pressure of the gas in state 2. P=F/a ∆P2= [250kg)(10m/s2)]/1.2x10-2m3)=2.04x103 Pa P2=P1+∆P2 P2=1.02x105Pa + 2.04x103Pa = 1.04x105 Pa b. Determine the volume of the gas in state 2. P1V1=P2V2 V2=(P1V1)/P2 = [(1.02x105Pa)(1.5x10-3m3)](1.04x105Pa) = 1.47x10-3m3 c. Indicate below whether the process from state 2 to state 3 is isothermal, isobaric, or adiabatic. _____Isothermal ___√__Isobaric _____Adiabatic Explain your reasoning. The transformation occurs at constant pressure d. Is the process from state 4 to state 1 isobaric? __√___Yes _____No Explain your reasoning. The pressure does not change during the transformation e. Determine the volume of the gas in state 4. V=? V1/T1=V2/T2 V4=V1T4/T1 = [(1.5x10-3m3)(373K)]/(273K) = 2.05x10-3m3 2003 B5. (10 points) A cylinder with a movable piston contains 0.1 mole of a monatomic ideal gas. The gas, initially at state a, can be taken through either of two cycles, abca or abcda, as shown on the PV diagram above. The following information is known about this system. Determine the change in internal energy, Ua – UC, between states a and c. ∆U=3/2nR∆T = 3/2 (0.1 mol)(8.314 J/mol•K)(721.7K-270.6K) = 562.5 J T=PV/nR = [(6x105Pa)(1.0x10-3m3)]/[(0.1 mol)(8.314 J/mol•K)] = 721.7K T=PV/nR = [(3x105Pa)(0.75x10-3m3)]/[(0.1 mol)(8.314 J/mol•K)] = 270.6K (b) i. Is heat added to or removed from the gas when the gas is taken along the path abc ? ______ added to the gas __√__removed from the gas ii Calculate the amount added or removed. From A to B pressure decreases while V is constant so Q is removed From B to C pressure is constant while work is done on the gas so Q is removed. ∆U = -Q+W for isobaric compression. 638 J = -(-562.5J) + (3x105 Pa)(0.25x10-3m3) (c) How much work is done on the gas in the process cda? W=p∆V = (6x105Pa)(0.25x10-3m3)= 150J (d) Is heat added to or removed from the gas when the gas is taken along the path cda? Heat is added to the gas because U increases as work is done. Explain your reasoning.