Energy & Power
advertisement

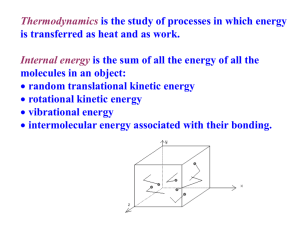
EE535: Renewable Energy: Systems, Technology & Economics Session 3: Energy Conversion 1 Energy Conversion Processes • A large array of energy conversion processes occur in nature • Man is capable of performing a number of addition processes using a variety of devices (or processes) • Usually more than one form of energy will emerge due to the action of a device • Many devices perform a number of conversion processes: e.g. : power plant Power Plant: Chemical -> heat -> mechanical -> electrical Energy Conversion Processes Initial Energy Form Chemical Radiant Electrical Mechancial Nuclear Heat Reactor Chemical Radiant Photolysis Electrical Electrolysis, Battery Charging Fuel Cell, Battery Discharge Burner, Boiler Photovoltaic cell Absorber Lamp, Laser Electric Motor Resistance, Heat Pump Mechanical Electric Generator, MHD Turbines Friction Heat Thermionic & thermoelectric generators Thermodynamic Engines Radiator Thermodynamics • First Law (Energy Conservation) – Energy can neither be created or destroyed – Energy can be utilized, not consumed • Second Law – The quality of a particular amount of energy, i.e. the amount of work or action that it can do, diminishes each time this energy is used. – We degrade or randomize energy • Energy we use is degraded into heat and then radiated out into space Entropy Low Entropy High Entropy 1kJ of Energy 1kJ of Energy Use Up Engine + Petrol Moves Waste heat Exhaust gases Mechanical energy (20%) Electrical Energy (Disordered energy has high entropy) Quality of energy in the universe is constantly diminishing Engines • Work can be changed into heat relatively easily – the reverse is a different challenge • From the second law of thermodynamics, we know that : – it is not possible to change completely into work, with no other change taking place • You cannot build a device that violates this law Simple Device - Piston p pressure weight W Vi Vf V Q T volume Removing weight from the piston permits the gas to expand The gas remains at constant temperature, absorbing heat Q from the reservoir The system follows the isotherm and does work W by lifting the weight The internal energy U (which depends only on temperature for an ideal gas), does not change during this isothermal expansion Simple Device - Piston • From first law of thermodynamics, Q=ΔU + W • In the piston example, the work W is exactly equal to the heat Q extracted from the reservoir – have we not just broken the second law of thermodynamics? • No – the system is not in the same state (volume and pressure changed) • To restore the system to its original condition, the piston must operate in a cycle – • A device that changes heat into work is called a Heat Engine Engine Cycle TH • During every cycle, heat QH is extracted from a reservoir at temperature TH • A portion is diverted to useful work W and the rest is discharged as heat Qc to a reservoir at temperature Tc • Because an engine operates in a cycle, U (gas) returns to its original value at the end of the cycle (ΔU = 0) • So, work done per cycle must equal net heat transferred per cycle: • |W| = | QH | -| Qc | engine QH W QC TC Efficiency • The purpose of an engine is to transform as much extracted heat QH into work as possible • We measure success of the engine by its thermal efficiency e – defined as the ratio of the work done per cycle: • e = |W| / |QH| = (|QH| - |QC|)/|QH| • In the case of (an impossibly) perfect engine, QC = 0 The Ideal Engine • There are no perfect engines • When considering gases, we are assuming an ideal gas – ignoring complexities of real gases • Assumed no friction, turbulence, unwanted heat transfer • System stays in thermal equilibrium at all times • Reversible process The Carnot Cycle • • Step 1 – Starting at (a), put the cylinder on a high temperature stand and remove weight and allow system to expand to (b) – Heat QH is absorbed by the system – Isothermal process –> U doesn’t change & added heat appears as work p pa a QH b pb Step 2 – From (b), put the piston on an insulating stand and remove weight and allow system to expand to (c), – This expansion is adiabatic as no heat enters or leaves the system – The system does work by moving the piston further and the temperature drops to Tc, as the energy to do the work must come from internal energy of the system W pd pc QC c d Va Vd Vb Vc V The Carnot Cycle • Step 3 – From (c), put the cylinder on a p colder heat reservoir – Increase weight to the piston and compress the gas to point (d) pa – Heat Qc is transferred from the gas to the reservoir – Compression is isothermal & work is done on the gas by the descending piston & its load a QH b pb • Step 4 – From (d), put the piston on an insulating stand and add weight and force system to compress back to (a), – This compression is adiabatic as no heat enters or leaves the system – Work is done on the gas and its temperature rises to Tc W pd pc QC c d Va Vd Vb Vc V The Carnot Engine • The special property of the Carnot engine is that its thermal efficiency can be written as: e = (TH – Tc)/ TH [Proof?] • i.e. the efficiency depends only on the temperatures of the two reservoirs between which it operates • No real engine operating between the same 2 temperatures can have an efficiency greater than a Carnot engine • The Carnot engine represents the limiting behavior of real engines • When run in reverse, the engine can be operated as a Carnot refrigerator, with a coefficient of performance given by: K = Tc/(TH – Tc) [Proof?] Efficiency • A consequence of the second law is that the perfect heat engine is impossible The efficiency of an ideal heat engine is: Efficiency e = 1 – T2/T1 Where T1 is the temperature of the heat source (hot gas produced by fuel combustion Where T2 is the surrounding air temperature For a car, T1 = 2400K, T2 = 300K So (Theoretical Efficiency) = 1 – 300/2400 = 0.88 Calorific Value • The calories of thermal units contained in one unit of a substance and released when the substance is burned • Energy Density What is the calorific value of petrol: = 10kWh/litre Note: density of petrol assumed to be 0.84kg/l Car Example Energy used per day = = Distance travelled per day X Energy per unit of fuel Distance per unit of fuel 50km/day 12km/litre X 10kWh/litre = 41.6 kWh/day What about the energy cost of producing the car’s fuel? What about the energy cost of manufacturing the car? Other Thermodynamic Cycles Cycle\Process Compression Heat Addition Expansion Heat Rejection. Power cycles normally with external combustion - or heat pump cycles Ericsson (First, 1833) Brayton adiabatic isobaric adiabatic isobaric (Reverse Brayton) adiabatic isobaric adiabatic isobaric Carnot isentropic isothermal isentropic isothermal Stoddard adiabatic isometric adiabatic isometric isothermal isometric isothermal isometric isothermal isobaric isothermal isobaric Bell Coleman Stirling Ericsson (Second, 1853) Power cycles normally with internal combustion Otto (Petrol) adiabatic isometric adiabatic isometric Diesel adiabatic isobaric adiabatic isometric Brayton (Jet) adiabatic isobaric adiabatic isobaric isobaric isometric adiabatic isobaric Lenoir (pulse jet) (Note: 3 of the 4 processes are different) Things to Consider • How much does a kWh of electricity cost a domestic user in Ireland? • Please investigate the use of a Stirling Engine in Solar – Thermal Applications