ELECTRON TRANSFER REACTIONS
advertisement

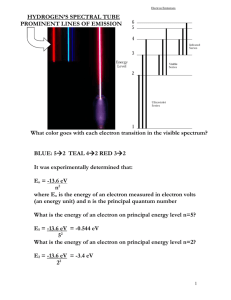
MODULE 27_03 ELECTRON TRANSFER REACTIONS Some Basic Principles Processes involving the transfer of electrons occur widely in the physical and life sciences. They range from simple exchange reactions in chemistry 2 3+ Feaq Feaq 3+ 2 Feaq Feaq to processes that drive energy storage and respiration in biological systems, such as the cytochrome-c/cytochrome oxidase couple which are both heme proteins. Cytochrome oxidase Cytochrome-c e Fe Fe oxidase Fe Fe In recent years, photochemical scientists have become keenly interested in electron transfer reactions because: (i) they occur as primary events in many photoprocesses. (ii) they can be conveniently studied using photophysical techniques (iii) photophysical methods offer excellent ways of testing the theories. Kinetic Aspects of Bimolecular Reactions An electron transfer reaction between individual molecules freely diffusing in a mobile liquid has characteristics of all such bimolecular reactions: kf D+A D+ + A - kr The two independent entities diffuse together, react, and two different entities diffuse apart. The overall process presumably proceeds via a collision complex, one or more reaction intermediates, or a transition state. Thus, micro-reversibility applies, and the process occurs on a continuous potential energy surface. No excited states have yet been invoked, so we can imagine the 1 process to be adiabatic (no crossings to other PE surfaces), although this concept is not always true. We can define rate constants for the forward and reverse processes, kf and kr, and an equilibrium constant, Keq. Students of electron transfer reactions are interested in determining values of these parameters and understanding the factors that influence them. The overall reaction, like all chemical reactions, will have characteristic G0, 0, and S0 parameters. In addition, since this is specifically electron transfer, we can relate G0 to reduction potentials, viz., G0 nFE 0 nF ( EA0 / A ED0 / D ) (27.1) However, thermodynamic constants are useful for describing equilibrium states only, not providing information on the mechanistic details. Experiment shows that for exoergic electron transfer processes, rate constant values occur over a wide range, up to the limit imposed by diffusion. It is of great interest to understand this variation. Let us consider a detailed scheme for the overall bimolecular process shown above. D+ + A- D+A kd k-d k-d e kn [DA] Precursor complex [DA] k-n kd k’-n # + - # [D+A-] [D A ] e k’n Reorganized precursor complex Successor complex 2 Reorganized successor complex A treatment using steady state approximation leads to 1 kobs (kd ka ) / kd ka (27.2) where ka under the condition that kd kn ve 2ve k n (27.3) k n k n When ka >> kd, we see that kobs = kd, i.e., the measured rate constant approaches the limit set by diffusion (see Module 24). Let us examine equation (27.3) in more detail (i) Under conditions when ve k n i.e., the electron moves to A more rapidly than the reorganized complex relaxes, then 1 1 ka kd kn kd k n K n 2 2 1 kd k n exp (Gn† / RT ) 2 (27.4) (27.5) where k-n is the rate of relaxation of vibrationally excited precursor complex, and Gn† is the energy barrier to nuclear reorganization. Thus ka is independent of e, the electron-hopping rate. (ii) On the other hand, when k n ve ka kd ve Kn (27.6) kd ve exp (Gn† / RT ) (27.7) Therefore in both cases (and all others), the nuclear reorganization process is a barrier to electron transfer and imposes an activation step. So we see that the overall (measured) rate constant is a combination of diffusion-dependent (kd) and activation-dependent (ka) terms: 1 kobs ka1 kd1 (27.8) As we saw above, when ka >> kd, then kobs = kd. This is a simplifying situation, but it means that our kinetic measurements can provide no information on the activation-dependent process because everything is limited by diffusion. However, the central segment of the sequence of processes 3 ( D / A) kn ( D / A) ve † k n ( D . / A. ) † ve occurs independently of how the sequence is initiated and if we wish to investigate its role in the sequence we need to circumvent the diffusion limiting problem. Later we will see how this can be done. For now, we assume that it can and proceed to examine ka. From the foregoing, it has emerged that the parameters k-n, e, and Gn† are important in determining the magnitude of ka. Theoreticians have examined these using classical mechanics (Marcus, Sutin, Hush) or semi-classical/quantum methods (Jortner, Levich). Gn† ;The Transition State Picture The partial scheme is ( D / A) precursor kn ( D / A) k n † reorganized precursor ve ve ( D / A ) † successor The steps prior to the formation of (D/A) and those after ( D / A ) are ignored in this argument. D and A may be polyatomic molecules, aquated metal ions, etc., and the reaction above proceeds with changes in bonding coordinates in D and A, and solvation around the complex ( D / A)† and ( D / A )† have identical nuclear configurations; they differ only in that a single electron has moved. The situation resembles a Franck-Condon type event, or even a radiationless transition between two states. Thinking of electron transfer in terms of a radiationless transition, one can write the expression k 2 e2 n (27.9) where is an average density of states in the acceptor. In electron transfer theory, this term usually appears as a Franck-Condon weighted density of states (FCWD). The electronic matrix 2 element term, e , contains the operator which drives the process. The classical picture due to Marcus (1956) is less rigorous and simpler, but provides useful physical insights. It also provides a comparison to the transition state theory (TST) of kinetics. Marcus chose to represent the complex multidimensional PE surfaces of polyatomic reactant pairs as a parabolic energy curve in "nuclear configuration space", see Figure 27.1. 4 20 PRODUCT STATE REACTANT STATE 15 10 G 5 G#n FIG. 30.1 0 4 2 0A 2 4 6 8 Nuclear configuration space The quantity is the energy required to move the electron in the reactant state (D/A) to the product state ( D / A ) without prior nuclear reorganization. In this it resembles a Franck- Condon event. The Gn# quantity represents the energy required to reconfigure the precursor 5 complex to a non-equilibrium nuclear configuration in which the electron transfer can occur and the system can switch from the reactant state curve to the product state surface. Note that Gn# . At the curve crossing, the electron can hop from one curve to another with some probability (rate). The situation shown in the schematic is for G 0 0 , i.e., an isoergic process. Analytical geometry of intersecting parabolas showed Marcus that Gn# / 4 in the isoergic condition. When (27.10) G 0 0 , then Gn# (G 0 ) 2 / 4 (27.11) # Thus the energy barrier (Gn ) to electron transfer depends on G 0 and in a quadratic manner. Figure 27.2 schematically demonstrates the effect on G#n as the value of G0 becomes increasingly negative. As the product parabola is lowered with respect to the red reactant curve, the nuclear reorganization barrier first becomes less and then increases again (the only change is in the overall driving force, there are no shape changes, nor does the curve minimum shift to left or right). l( x) k( x) 25 20 0 G0 20 FIG. 27.2 40 10 5 0 5 6 10 15 † † Earlier we showed ka exp(Gn / RT ) or ln ka Gn / RT . Thus, Marcus theory predicts that for weakly exoergic reactions log ka increases as G 0 increases, maximizes at G 0 , and decreases again as G 0 increases beyond . This is a remarkable result, flying in the face of expectation and it led to Marcus’s Nobel Prize in Chemistry in the early 1990s. Figure 27.3 G E shows the graph of log ka vs. inkvolts E . a exp R T 110 13 13 1 10 1 10 12 normal region inverted region ka k( E 0.5) 1 10 11 1 10 10 Figure 27.3 1 10 9 1 10 9 2 0 3 2 E 4 4 In the foregoing development we 20 have assumed that the product state is formed in its zeroth 10 vibrational state. However, this is a simplification and in fact the 0 formation of product species in vibrational 10 above the zeroth is very possible. FIG. 27.4 20 states 10 5 In Figure 27.4 the family of 0 5 10 15 dashed curves represent four vibrational 7 energies of the product state. Whereas the red dashed curve (v = 0) crosses the reactant curve in the inverted region, the higher vibrational modes do not. The rate constant observed will be a weighted sum of the contributions from all the modes, and it will be larger than that if the v = 0 mode was the only contributor. Thus the inverted region will be less pronounced than otherwise; the parabola will depart from the symmetrical form shown in Figure 27.3. The Marcus approach, being geometrical, assumes symmetrical sets of pure parabolas, i.e., there is only very weak interaction at the crossing point. Thus the reaction is by necessity non-adiabatic, because curve crossing must occur. Figure 27.5 shows the adiabatic/non-adiabatic situation FIG. 30.5 We can see that at the crossing point the two curves are degenerate and under such a condition time dependent perturbation theory instructs us that the probability of transferring to the product curve will be proportional to sin 2 V t . The vibrational motion of the nuclei in the reactant state will be such that it spends only a short time in the crossing region. Thus for states where V is small (< kBT), the probability of reaction will be small and the system will continue on the reactant surface for many passages through the crossing region. On the other hand, when the perturbation is large (> kBT), as for the adiabatic case, the argument of the sin2 term is large and the oscillatory frequency will be sufficient to ensure easy passage into product space. The reorganization energy, The barrier to electron transfer, according to Marcus' model, is manifest as a free energy term composed of G 0 and components. The former is a state property, which defines the overall 8 free energy changes in going from reactants to products. The quantity is an energy term that describes the reorganization of nuclear configurations that are needed to allow the electron to hope form one parabola to another. Energy requirements due to structural adjustments within the molecular frameworks of the D and A species (inner sphere) and readjustment of solvent dipoles to accommodate the charge shift (outer sphere) are included in , thus s 0 where (27.12) s depends on solvent motions and 0 depends on internal nuclear adjustments (bond lengths, and so on.) Marcus (1959) arrived at an expression for s on the idea that a solvent behaves as dielectric continuum (no local structure; hard sphere molecules). s e2[1/ 2rD 1/ 2rA 1/ RDA ]( Dop1 Ds1 ) (27.13) where rD , rA are the radii of donor and acceptor, RDA is the separation of D and A, and Dop , DS are the optical and static dielectric constants of the solvent For polar organic liquids ( CH 3CN ) s 0.75 eV For non-polar organic liquids ( C6 H12 ) s 0.15 eV The value of 0 is not easily calculated, but it is usually estimated from considerations of the force constants of normal mode vibrations in the reactant and product species. A typical value for is ca 0.4 eV. The ve parameter: ve has been used to represent the frequency (or rate) at which an electron can shift between (D/A)# and (D+ /A- )# . This can be broken down ve vn el vn (27.14) is the frequency with which the reorganized nuclear configuration is reached and el is the probability of electron transferring from one PE curve to another in the reorganized configuration. 9 In TST terms, vn can be regarded as being similar to an entropy-controlled term, viz., vn k BT exp (S # / R ) h (27.15) The maximum value of n is kBT/h, which is approximately 1013 s-1 when S# = 0. el is similar to the transmission coefficient of TST and can take values between zero and one. It represents the effectiveness (probability) of the electron switching its molecular identity with the concomitant change of the system from the reactant surface to the product surface. Thus, it depends on the interaction energy between the two curves (surfaces) at the crossing region-see Figure 27.5 and the preceding discussion. Thus el V , where V is the electronic coupling 2 matrix element (equivalent to e used earlier). Molecular wave functions decrease exponentially with distance from their maximum amplitude. Thus, the overlap increases as the distance between the reacting species decreases (Figure 27.6) FIG. 27.6 It is found that V exp ( r RDA ) , where r (not to be confused with e ) is a multiplier of RDA having dimensions of m-1 and can be thought of as being equivalent to a molecular resistance. If r is small for a given effectively; if RDA , then V is large and electron transfer occurs r is large for a given RDA , then V is less and the transfer efficiency is reduced. Thus, we see that the overall rate constant for electron transfer in the activated case (ka) depends on (i) distance between the participants (ii) the frequency with which nuclei reorganize (iii) a reorganization barrier 10 Overall the activated rate constant can be described by ka vn el exp[(G 0 ) 2 / 4 RT ] (27.16) The maximum value of n is approximately 1013 s-1. The electronic coupling factor, Kel, depends on RDA and on the relative orientation between the dipoles in the donor and acceptor. The parameter is the reorganization energy, which depends on solvent reorientation and on changes Figure 27.7 shows a 3-D plot of ln k vs and G internal to the molecules. FIG.27.7 20 18 ln k 16 14 12 10 8 30 20 10 -10 -5 0 5 10 15 20 25 30 35 40 G Note that nothing in the foregoing has required photoexcitation. The development has been quite independent of the electronic state of the reacting species. Reviews and Literature Entries 1. G. L. Closs and J. R. Miller. Science (1988), 240, 440-447 2. G. McLendon. Acc. Chem. Res. (1988), 21, 160-167 3. R. A. Marcus and N. Sutin. Biochem. Biophys. Acta (1984), 811, 265-322. 11 4. M. D. Newton and N. Sutin. Ann. Rev. Phys. Chem. (1984), 35, 437-80. 12