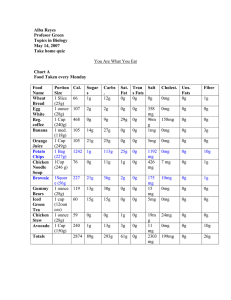
Know the following terms: Nuclear Transmutation Fission Fusion
advertisement

Name:__________________________________________ Know the following terms: Nucleon Nuclide Radioisotope Nuclear Transmutation Fission Fusion Half-Life Complete the following nuclear reactions: 1. 2. 3. 4. 5. 6. 7. 27 13 30 Al 24He 15 P _____________ 31 15 32 P 12H 15 P _______ 35 Cl ________ 16 S 37 17 2 1 H _________ 01 n 31 15 14 7 28 P 11H 14 Si _______ N ______ 178 O 11H 43 19 43 K 20 Ca _____ 8. 13 H 23He ______ 9. 36 Li 01n 24 He ________ 0 10. 214 82 Pb 1 ________ 11. 2963Cu 12H 13H _______ 12. 36 Li 11H 24 He _________ 13. ________ 237 93 Np 14. 49 Be 36 Li _______ Finish the following equations and determine which is Fission and which is Fusion? (be sure to use your definition from above) Fission/Fusion 11 4 14 1. 5 B 2 He 7 N _____________ ___________________________ 95 U 01n42 Mo 201n ______ ___________________________ Pu n _______ ___________________________ 38 Cu 11H 17 Cl 01n ______ ___________________________ 235 92 2. 3. 239 94 4. 63 29 Name of Decay Alpha Beta Gamma Neutron 1 0 Symbol for Decay (both Greek letter and A nuclear symbol) Z X Decay’s Effect on the nucleus Mass No. & Atomic No. Effects Finish the following equations and determine the type of decay or emission that occurs. (α, β, or γ) 1. What type of decay or emission occurs when 23490Th* decays into 23490Th ? *see notes.(show the reaction) 2. What type of decay or emission occurs when 23892U decays into 23490Th ? (show the reaction) 3. What type of decay or emission occurs when 3014Si decays into 3015P ? (show the reaction) Solve the following Half-Life problems: 1. The half-life of Polonium-210 is 138.4 days. How many milligrams of Polonium-210 remain after 415.2 days if you started with 2.0mg of the isotope? 2. Assuming a half-life of 1599 years, how many years will be needed for Radium to decay to 1/16 of the original 16/16? 3. A sample contains 16mg of Polonium-218. After 12 min., the sample will contain 1.0mg of Polonium-218. What is the half-life of Polonium-218? 4. A sample contains 4.0mg of Uranium-238. After 4.46 x 109 years, the sample will contain 2.0mg of Uranium-238. What is the half-life of Uranium-238? 3 5. Try to fill in the lines for each using 210 80 Hg 222 86 Rn particle removed Rn 1 α, β, γ, 0 n , particles or an isotope ( ZA ). particle added 216 86 197 79 Au 197 79 Au 200 78 Pt 204 80 Hg