Density ws 3A
advertisement

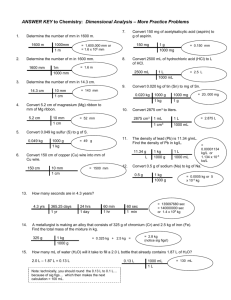
Density Worksheet 3A NAME _______________________ DATE ___________ PERIOD_____ Calculate the density using the information given. Follow SIG DIG rules to round off your final answer. SHOW YOUR WORK!!! 1. Mass = 41.80 g Volume = 6.00 mL Density =_______________ 2. Mass = 67.00 g Volume = 8.97 mL Density =_______________ 3. Mass = 38.04 g Volume = 81.00 mL Density =_______________ 4. Mass = 50.80 g Volume = 600.0 mL Density =_______________ Calculate the volume using the information given. Follow SIG DIG rules to round off your final answer. SHOW YOUR WORK!!! 5. Mass = 71.02 g Density = 0.078 g/mL Volume = _______________ 6. Mass = 712.60 g Density = 20.01 g/mL Volume = _______________ 7. Mass = 26.00 g Density = 9.01 g/mL Volume = _______________ 8. Mass = 78.00 g Density = 504.2 g/mL Volume = _______________ Calculate the mass using the information given. Follow SIG DIG rules to round off your final answer. SHOW YOUR WORK!!! 9. Volume = .06 mL Density = 20.80 g/mL Mass = ______________ 10. Volume = 290.00 mL Density = .0807 g/mL Mass = ______________ 11. Volume = 6.40 mL Density = 51.06 g/mL Mass = ______________ 12. Volume = .03 mL Density = 20.10 g/mL Mass = ______________ 13. Enter the data into the data table and then calculate the mass and then the density of the liquid. Show your work and round your answers off following SIG DIG rules. Mass of grad = 32.50 g Mass of grad and liquid = 38.75 g Volume of liquid = 6.90 mL Mass of Grad (g) Mass of Grand & Liquid (g) Mass of Liquid (g) Volume (mL) Density g/cm3) 14. Three clear colorless liquid have the following densities: water - 1.00 g/cm3 methyl alcohol - 0.79g/cm3 methyl acetate – 0.93 g/cm3 If 10 mL of each of these liquids were placed in a graduated cylinder, which would be On the bottom? In the middle? On top?