Chemistry Dimensional Analysis Answer Key
advertisement
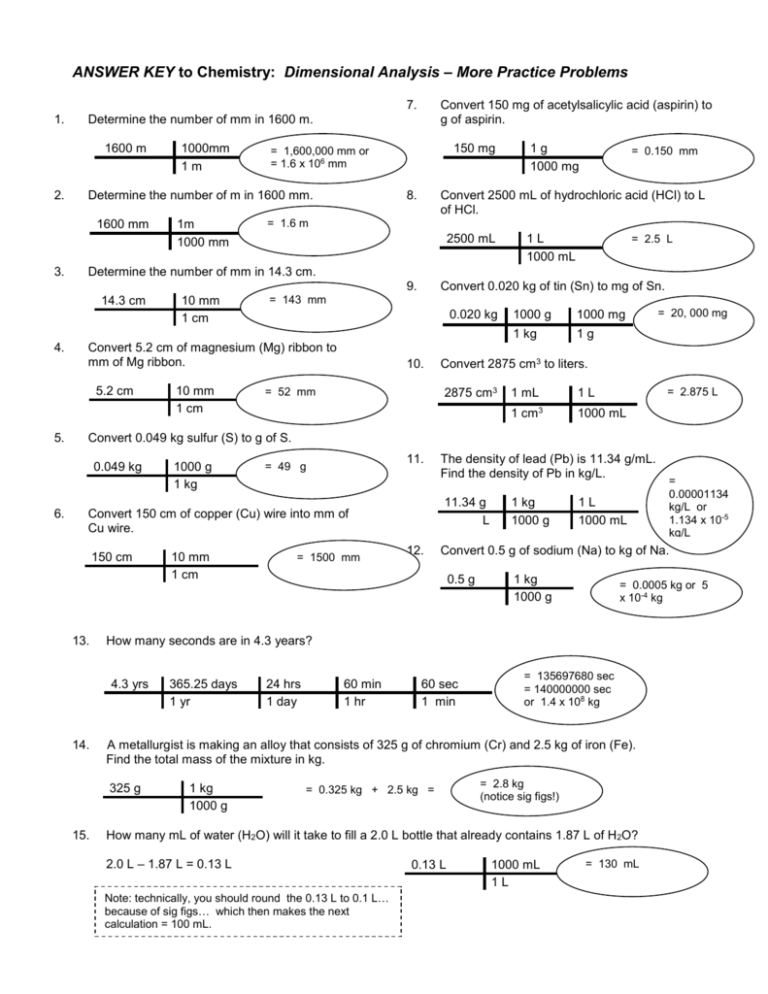
ANSWER KEY to Chemistry: Dimensional Analysis – More Practice Problems 7. 1. 1600 m 2. 1000mm 1m 1m 1000 mm 150 mg = 1,600,000 mm or = 1.6 x 106 mm Determine the number of m in 1600 mm. 1600 mm 3. Convert 150 mg of acetylsalicylic acid (aspirin) to g of aspirin. Determine the number of mm in 1600 m. 8. Convert 2500 mL of hydrochloric acid (HCl) to L of HCl. = 1.6 m 2500 mL 9. 1L 1000 mL = 2.5 L 10 mm 1 cm Convert 0.020 kg of tin (Sn) to mg of Sn. = 143 mm 0.020 kg 10. 1000 mg 1 kg 1g = 20, 000 mg Convert 2875 cm3 to liters. 2875 cm3 = 52 mm 1000 g = 2.875 L 1 mL 1L 1 cm3 1000 mL Convert 0.049 kg sulfur (S) to g of S. 0.049 kg 6. 10 mm 1 cm Convert 5.2 cm of magnesium (Mg) ribbon to mm of Mg ribbon. 5.2 cm 5. = 0.150 mm Determine the number of mm in 14.3 cm. 14.3 cm 4. 1g 1000 mg 1000 g 1 kg 11. = 49 g 11.34 g L Convert 150 cm of copper (Cu) wire into mm of Cu wire. 150 cm 13. 12. 1L 1000 mL 365.25 days 1 yr 0.5 g 24 hrs 1 day 60 min 1 hr 1 kg 1000 g 60 sec 1 min = 0.325 kg + 2.5 kg = 1 kg 1000 g = 0.0005 kg or 5 x 10-4 kg = 135697680 sec = 140000000 sec or 1.4 x 108 kg = 2.8 kg (notice sig figs!) How many mL of water (H2O) will it take to fill a 2.0 L bottle that already contains 1.87 L of H2O? 2.0 L – 1.87 L = 0.13 L Note: technically, you should round the 0.13 L to 0.1 L… because of sig figs… which then makes the next calculation = 100 mL. = 0.00001134 kg/L or 1.134 x 10-5 kg/L Convert 0.5 g of sodium (Na) to kg of Na. A metallurgist is making an alloy that consists of 325 g of chromium (Cr) and 2.5 kg of iron (Fe). Find the total mass of the mixture in kg. 325 g 15. = 1500 mm 1 kg 1000 g How many seconds are in 4.3 years? 4.3 yrs 14. 10 mm 1 cm The density of lead (Pb) is 11.34 g/mL. Find the density of Pb in kg/L. 0.13 L 1000 mL 1L = 130 mL











