QUANTUM NUMBERS
advertisement

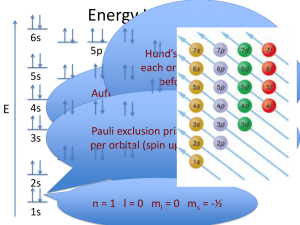
AP Chemistry Summary: Quantum Numbers By solving the Schrödinger equation we obtain a set of mathematical equations, called wave functions (), which describe the state of the electrons about the nucleus. Squaring the wave function (2) for an electron in an atom gives an atomic orbital; this atomic orbital describes a region of space in which there is a high probability of finding the electron. Energy changes within an atom are the result of an electron changing from a wave pattern with one energy to a wave pattern with a different energy (usually accompanied by the absorption or emission of a photon of light). Each electron in an atom is described by four different quantum numbers. The first three (n, l, ml) specify the particular orbital of interest, and the fourth (ms) specifies how many electrons can occupy that orbital. 1. Principal Quantum Number (n): n = 1, 2, 3, …, ∞ . Specifies the energy of an electron and the size of the orbital. All orbitals that have the same value of n are said to be in the same energy level. For a hydrogen atom with n=1, the electron is in its ground state; if the electron is in the n=2 orbital, it is in an excited state. The total number of electrons in a given energy level is given by 2n2. The total number of orbitals for a given n value is n2. For “A” group elements, the value of n for the last electron to enter the atom is given by the row number ( for Mg, n=2 for the last electron ) For “B” group elements, the value of n for the last electron to enter the atom is given by the row number – 1 (for Fe, n=3) For Lanthanides and Actinides, the value of n for the last electron to enter the atom is given by the row number – 2 (for U, n=5) 2. Orbital Quantum Number (l): l = 0, ... , n - 1. Specifies the shape of an orbital with a particular principal quantum number. The orbital quantum number divides the energy level into smaller groups of orbitals called sublevels. Usually, a letter code is used to identify l to avoid confusion with n: l = 0, s sublevel l = 1, p sublevel l = 2, d sublevel l = 3, f sublevel l = 4, g sublevel The sublevel with n=2 and l=1 is the 2p sublevel; if n=3 and l=0, it is the 3s sublevel, and so on. The value of l also has a slight effect on the energy of the sublevel; the energy of the sublevel increases with l (s < p < d < f). 3. Magnetic Quantum Number (ml): ml = -l, ..., 0, ..., +l. Specifies the number of orbitals in each sublevel (often referred to as the orientation in space of an orbital) of a given energy (n) and shape (l). This number divides the sublevel into individual orbitals which hold the electrons; the above equation gives the number of orbitals in each sublevel given by l. Thus ... If l = 0 (s sublevel), ml = 0 (one value) – there is one s orbital in an s sublevel If l = 1 (p sublevel), ml = -1, 0, 1 (three values) – there are 3 p orbitals in a p sublevel If l = 2 (d sublevel), ml = -2, -1, 0, 1, 2 (five values) – there are 5 d orbitals in a d sublevel If l = 3 (f sublevel), ml = -3, -2, -1, 0, 1, 2, 3 (seven values) – there are 7 f orbitals in a f sublevel Table of Allowed Quantum Numbers Number of Orbital Number of orbitals Name electrons 1 1s 2 n 1 l 0 ml 0 2 0 0 1 2s 2 1 -1, 0, +1 3 2p 6 0 0 1 3s 2 1 -1, 0, +1 3 3p 6 2 -2, -1, 0, +1, +2 5 3d 10 0 0 1 4s 2 1 -1, 0, +1 3 4p 6 2 -2, -1, 0, +1, +2 5 4d 10 7 4f 14 3 4 3 -3, -2, -1, 0, +1, +2, +3 4. Spin Quantum Number (ms): ms = +½ or -½. Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions (sometimes called up and down). All of the above information essentially summarizes into the following table below: